What is the Induced Pluripotent Stem Cells Market Size?
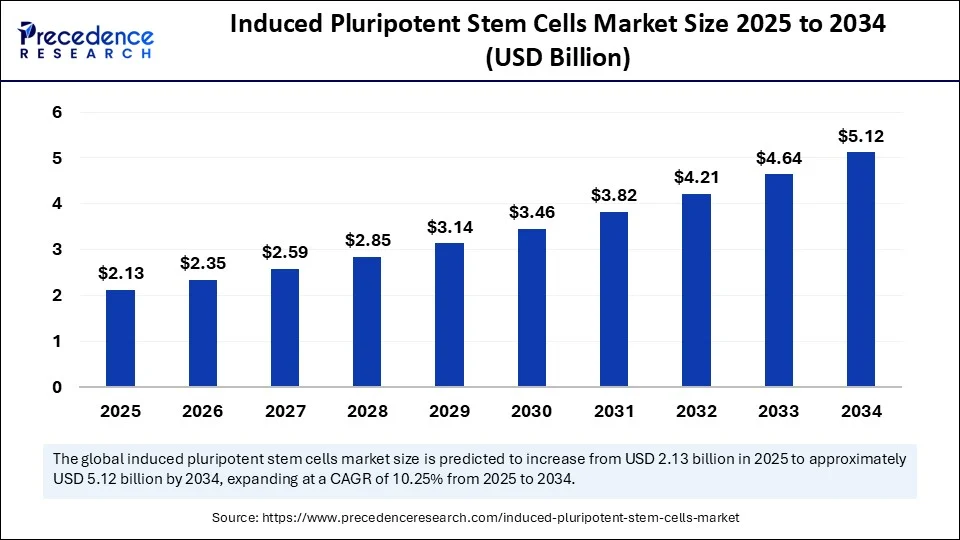
The global induced pluripotent stem cells market size is calculated at USD 2.13 billion in 2025 and is predicted to increase from USD 2.35 billion in 2026 to approximately USD 5.12 billion by 2034, expanding at a CAGR of 10.25% from 2025 to 2034. The market growth is attributed to the increasing demand for patient-specific cell therapies and disease models driven by advancements in reprogramming technologies and regenerative medicine.
Induced Pluripotent Stem Cells MarketKey Takeaways
- In terms of revenue, the global induced pluripotent stem cells market was valued at USD 1.93 billion in 2024.
- It is projected to reach USD 5.12 billion by 2034.
- The market is expected to grow at a CAGR of 10.25% from 2025 to 2034.
- North America dominated the global induced pluripotent stem cells market with the largest share of 36% in 2024.
- Asia Pacific is expected to grow at a notable CAGR from 2025 to 2034.
- By application, the drug discovery & toxicology testing segment held the major market share of 36% in 2024.
- By application, the disease modelling segment is projected to grow at a CAGR between 2025 and 2034.
- By cell type/lineage, the hematopoietic cells segment contributed the biggest market share in 2024.
- By cell type/ lineage, the hepatocytes segment is expanding at a significant CAGR between 2025 and 2034.
- By product & services, the iPSC-derived cells segment captured the highest market share of 41% in 2024.
- By product & services, the stem cell banking services segment is expected to grow at a significant CAGR over the projected period.
- By end user, pharmaceutical & biotech companies segments generated the major market share of 44% in 2024.
- By end user, the CROs & CDMOs segment is expected to grow at a notable CAGR from 2025 to 2034.
- By technology, non-integrating methods dominated the global induced pluripotent stem cells market in 2024 and are expected to sustain the growth in the coming years.
Artificial Intelligence: The Next Growth Catalyst in Induced Pluripotent Stem Cells
Artificial intelligence (AI) is a game-changer in the induced pluripotent stem cell (iPSC) market as it improves research productivity, scalability, and accuracy in therapeutic development. The algorithms of AI guide the reprogramming of adult cells into iPSCs to optimize, reduce errors, and enhance the reproducibility in companies and research institutions. Furthermore, the further advance of AI opens the prospects of clinical application scaling opportunities and promotes the worldwide expansion of iPSC-based technologies.
Strategic Overview of the Global Induced Pluripotent Stem Cells Industry
The induced pluripotent stem cells market comprises products and services related to stem cells that are genetically reprogrammed from adult somatic cells to an embryonic-like pluripotent state. These cells can differentiate into any cell type, making them a powerful tool in regenerative medicine, drug discovery, disease modeling, toxicology screening, and cell-based therapies. Unlike embryonic stem cells, iPSCs avoid ethical concerns and can be patient-specific, enabling personalized medicine and immune-compatible therapies. Market growth is fueled by advances in cell engineering, automation, CRISPR technology, and increasing R&D investment in cell-based therapeutics.(Source:https://www.eurogct.org)
Despite existing innovations, the increased incidence of chronic illnesses and genetic problems is contributing to an increasing global need for advanced regenerative therapies. Induced pluripotent stem cell (iPSC) technology is becoming one of the most vital focuses of biomedical research and innovation, as they are cells derived from an already present adult somatic cell and reprogrammed into a pluripotent form. The National Institutes of Health (NIH) reported that in 2024, there were more than 100 active clinical trials using iPSC-derived products, including Parkinson's treatment, macular degeneration, and cardiac repair. The California Institute of Regenerative Medicine (CIRM) is a government-sponsored program that, along with other initiatives, has statistics devoted to scores of iPSC-related projects aiming at taking laboratory breakthroughs to the clinic. Furthermore, with personalized medicine being the latest trend in health care, further investments in iPSC systems will drive the market in the coming years.(Source:https://www.nih.gov)
What are the Growth Factors in the Induced Pluripotent Stem Cells Marke
- Rising Demand for 3D Disease Modeling: Growing preference for physiologically relevant models is fuelling the adoption of iPSC-derived 3D organoids in preclinical research.
- Boosting Applications in Drug Toxicity Testing:iPSCs are driving safer pharmaceutical development by enabling early-stage human-specific toxicity screening without animal reliance.
- Expanding Use in Rare Disease Research: Increasing focus on rare and orphan diseases is propelling demand for iPSC models tailored to niche genetic conditions.
- Driving Innovation in Organoid Technology: Advancements in iPSC-based organoid systems are enhancing in vitro replication of complex tissues, supporting precision medicine.
Market Outlook:
- Market Growth Overview: The Induced Pluripotent Stem Cells market is expected to grow significantly between 2025 and 2034, driven by the rise of regenerative medicine and cell therapy, expansion in drug development and disease modeling, and advancements in production technologies.
- Sustainability Trends: Sustainability trends involve waste reduction and lifecycle management, green lab practices and energy efficiency, and ethical and responsible materials sourcing.
- Major Investors: Major investors in the market include Fujifilm (via Fujifilm Cellular Dynamics, Inc.), Thermo Fisher Scientific Inc., Bayer, Astellas Pharma Inc., and Lonza Group AG.
- Startup Economy: The startup economy is focused on automation and advanced manufacturing, AI and data-driven drug discovery, and niche disease modeling and diagnostics.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 5.12 Billion |
| Market Size in 2025 | USD 2.13 Billion |
| Market Size in 2026 | USD 2.35 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 10.25% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Application, Cell Type / Lineage, Product & Service, End User,Technologyand Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
How Is the Increasing Prevalence of Chronic and Genetic Disorders Accelerating Demand in the Induced Pluripotent Stem Cells Market?
The increasing presence of endocrine disruptors, obesity, and related co-morbidities, and an increasing number of carcinogens in the environment are damaging DNA structures, leading to a rise in both genetic and chronic conditions. These factors have driven the induced pluripotent stem cells market in the last few years. The rise in incidences of these diseases is likely to fuel the market demand involving superior cell-based models and custom-made therapies. According to the CDC's 2024 report, approximately 60% of Americans live with at least one chronic disease, while 40% are affected by two or more chronic conditions. Increasing instances of diseases, such as Parkinson's, Alzheimer's, diabetes, and several rare genetic diseases, have induced investigators and drug companies to examine patient-derived iPSC lines to execute precise modeling and regenerative efforts. (Source: https://web.brc.riken.jp)
These cells are an ethically acceptable, genetically matched source of cells to study the disease progression and also to assess response to drugs by increasing the preclinical success rates. The use of iPSC platforms is a topic that is gaining traction in hospitals and research centers so that they can conduct clinical tests that seek to provide long-term solutions rather than symptomatic ones. In 2024, the RIKEN Center for Biosystems Dynamics Research in Japan went further to develop its iPSC platform by adding CRISPR-Cas9 gene editing to its platform to study inherited retinal diseases to further strengthen the use of iPSCs in the personalized development of therapy. Furthermore, such a change in order to disease-modifying solutions is further reinforcing the research and development base of the induced pluripotent stem cells market and further fuelling its growth.(Source:https://www.cdc.gov)
Restraint
How has Regulatory Complexity Slowed Therapeutic Approval in Clinical-Grade iPSC Applications?
High costs associated with iPSC development and manufacturing are anticipated to hinder the market. The specialized infrastructure, specialized personnel, and high degree of quality control involved in establishing the iPSC lines under GMP conditions introduce barriers to entry. Such expenses limit entry to well-capitalized academic hubs and giant biotech companies, with the minor stakeholders left with reduced choices of licensing or joint ventures. Furthermore, the regulatory complexity surrounding clinical-grade iPSC applications is expected to limit the pace of therapeutic approvals, thus further hampering the induced pluripotent stem cells market.
Why Is The High Investment In Stem Cell Research And Regenerative Medicine Reshaping The Technological Roadmap Of The Induced Pluripotent Stem Cells Market?
Growing investment in stem cell research and regenerative medicine is anticipated to create immense opportunities for the market. Governments, universities, and private corporations have increased funding efforts on a great scale to hasten the discovery and commercialization of iPSCs. Nations, such as the U.S., Japan, and the UK, offer large grants to work on translational studies, and the biotech companies join hands with universities to scale up the protocols, started in laboratories, to clinical grades.
In Canada, the Centre for Commercialization of Regenerative Medicine (CCRM) opened a new venture lab in 2024 to assist early-stage ventures in doing work on iPSC production and clinical development. Furthermore, the increase in financial and infrastructural grants by the government and private organizations to facilitate innovation in these technologies further fuels the induced pluripotent stem cells market in the coming years. (Source: https://www.ccrm.ca)
On February 23, 2024, the California Institute for Regenerative Medicine (CIRM)—the world's largest organization focused on regenerative medicine—allocated $56 million to advance clinical research targeting Parkinson's disease, autoimmune disorders, and various cancers. The funding supports seven projects under CIRM's clinical program, which facilitates the progression of stem cell and gene therapy-based therapies through every phase of clinical trial development.
(Source:https://www.cirm.ca.gov)
Application Insights
Why Do Drug Development and Toxicity Testing Lead the Application Landscape in the Induced Pluripotent Stem Cells Market?
The drug discovery and toxicology testing segment dominated the induced pluripotent stem cells market in 2024, accounting for an estimated 36% market share, due to the increased need for valid, relevant, and predictive in vitro models to determine the efficacy and safety of drugs before clinical trials. Furthermore, this enhanced the company as the value of the iPSCs as patient-specific, scalable cell sources that gave them the edge in the induced pluripotent stem cells market.
The disease modeling segment is expected to grow at the fastest rate/fastest CAGR in the coming years, owing to the need for patient-specific models of complex and rare diseases. Such a segment acquires impetus through the fact that iPSCs have the potential to recapitulate the disease phenotype in a cellular model, which allows the accurate study of disease processes and pathogenesis.
Biotechnology companies and research centers are putting huge investments in iPSC-based frameworks to investigate neurodegenerative disorders, cardiovascular abnormalities, and genetic disorders. To aid iPSC-based disease modeling, NIH and other research organizations around the world have also raised their funding on the subject, particularly on disease conditions that are unlikely to be resolved using effective treatments. In 2024, the Harvard Stem Cell Institute partnered with Vertex Pharmaceuticals to model cystic fibrosis with iPSC-derived lung epithelial cells to generate data published in Nature Medicine. Furthermore, the emergence of the souped-up, personalized medicine market and the development of CRISPR genome editing further facilitate the segment.(Source: https://investors.vrtx.com)
Cell Type / Lineage Insights
What Drives the Dominance of Cardiomyocytes in the Induced Pluripotent Stem Cells Market by Cell Lineage?
Hematopoietic cells segment held the largest revenue share in the induced pluripotent stem cells market in 2024, due to the widespread application of using them in regenerative medicine, immunotherapy, and modelling of haematological disease. The iPSC-derived cells find wide application in the study of blood-related diseases, such as leukaemia, anaemia, and immunodeficiencies, as they recapitulate the stages of the native haematopoiesis.
Hematopoietic cells are of interest to researchers and biopharmaceutical developers due to their applicability in the generation of a number of different immune cells, such as T cells and natural killer (NK) cells. They are more frequently being studied in off-the-shelf cell therapies. In 2024, the NIH and the California Institute for Regenerative Medicine (CIRM) invested more heavily in the study of iPSC-derived blood cell products. Furthermore, the new directions in differentiation procedures, the large-scale production of hematopoietic cells using GMP processing, likely continue to fuel the hematopoietic cells segment.
(Source: https://pmc.ncbi.nlm.nih.gov)
Hepatocytes segment is expected to grow at the fastest rate/fastest CAGR in the coming years, owing to the increasing need for liver disease modeling, toxicity testing, and accurate drug screening. Such liver cells, differentiated by iPSCs, are being used more to investigate nonalcoholic steatohepatitis (NASH), viral hepatitis, and inherited metabolic disorders since they are able to recapitulate important hepatic functions. Moreover, the hepatocytes offer scalability, reproduction, and greater metabolic fidelity, further propelling the growth of this segment.
Product & Service Insights
What Makes iPSC-Derived Cells the Dominant Offering in the Induced Pluripotent Stem Cells Market?
iPSC-derived cells segment dominated the induced pluripotent stem cells market in 2024, accounting for 41% market share, due to their prominent use in drug research, disease modeling, regenerative medicine, and toxicity screening, creating massive demand in both research and clinical scenarios. It is the ability to produce different lineages of tissues that are considered useful, as the chances of generating functional relevance of models of complex tissue in humans. Furthermore, the superior predictive validity of iPSC-derived hepatocytes compared to traditional models in drug-induced liver injury (DILI) modelling further boosts the segment in the coming years.
The stem cell banking services segment is expected to grow at the fastest CAGR in the coming years, owing to the increased awareness of customized regenerative medicine and the lifelong benefit of an autologous iPSC repository. Institutions increase their banking facilities to meet rising demands regarding safe and GMP-compliant storage of patient-specific iPSC lines. These services provide access to genetically stable cell lines that have been quality-controlled and can be used in future therapeutic applications of neurology, oncology, and rare diseases. Moreover, the California Institute of Regenerative Medicine (CIRM) also invested in a statewide infrastructure to facilitate the use of iPSC biorepositories, further facilitating the segment.
End User Insights
How Are Pharmaceutical & Biotechnology Companies Driving the Induced Pluripotent Stem Cells Market Forward?
Pharmaceutical & biotechnology companies segment held the largest revenue share in the induced pluripotent stem cells market in 2024, accounting for 44% of the market share, due to the vast use of iPSCs in their drug discovery pipeline, drug toxicity assessment, and regenerative therapies pipeline. To increase target identification and to enhance predictive toxicology, more companies are embracing the use of iPSC-derived cellular models to reduce loss during major clinical trials that have a high failure rate. Additionally, the current evolution towards human-relevant preclinical models is expected to fuel the segment.
The CROs & CDMOs segment is expected to grow at the fastest rate in the coming years, as they play a central role in the upscaling of iPSC applications into commercial and translational applications. The growing outsourcing by drug and biotech companies to cut R&D expenses and improve time-to-market is compelling the pharmaceutical industry towards CROs and CDMOs that provide specialized iPSC services.
Lonza and Pluristyx extended their iPSC manufacturing capabilities, such as the GMP-grade cell lines development and cryopreservation services for personalized regenerative therapy in 2024. The rest of these organizations are expected to increase their momentum further since they assist in early clinical initiatives, genome editing services, and custom differentiation procedures. In 2025, the Mayo Clinic Center for Regenerative Medicine and a U.S.-based CDMO will contract to produce iPSC-derived dopaminergic neurons to a clinical grade. Furthermore, the new guidelines regarding the commercialization-grade iPSC production further facilitated the segment in the coming years.(Source:https://www.cira-foundation.or.jp)
Technology Insights
What Positions Non-Integrating Methods as the Preferred Reprogramming Technology in the Induced Pluripotent Stem Cells Market?
Non-integrating methods segment dominated the global induced pluripotent stem cells market in 2024 and is expected to sustain the growth in the coming years, as it eliminates insertional mutagenesis and preserves genomic fitness. The most popular of them are episomal vectors, Sendai virus-based reprogramming, and mRNA transfection, implemented widely in the research and clinical pipeline. This strategy should work to enhance the rate of clinical translation, especially in the area of cell therapy and regenerative medicine, with safety and reproducibility that fits the regulatory requirements of the genome. (Source: https://www.cirm.ca.gov)
In 2024, scientists in the Stanford Institute for Stem Cell Biology and Regenerative Medicine reported increased success rates with synthetic modified mRNA-based reprogramming, with reports of increased consistency in the quality of iPSC across neurological and cardiovascular disease models. NIH Stem Cell Program in its 2024 iPSC roadmap, identified an increasing interest in adopting xeno-free, feeder-free, and integration-free methods of reprogramming that simplify downstream differentiation and potential therapeutic uses. Furthermore, regulators such as the European Medicines Agency (EMA) are marshaling to harmonize frameworks of GMP to promote integration-free, clinically validated iPSC production protocols, thus further fuelling the segment. (Source: https://www.asgct.org)
(Source: https://pubmed.ncbi.nlm.nih.gov)
Regional Insights
U.S. Induced Pluripotent Stem Cells Market Size and Growth 2025 to 2034
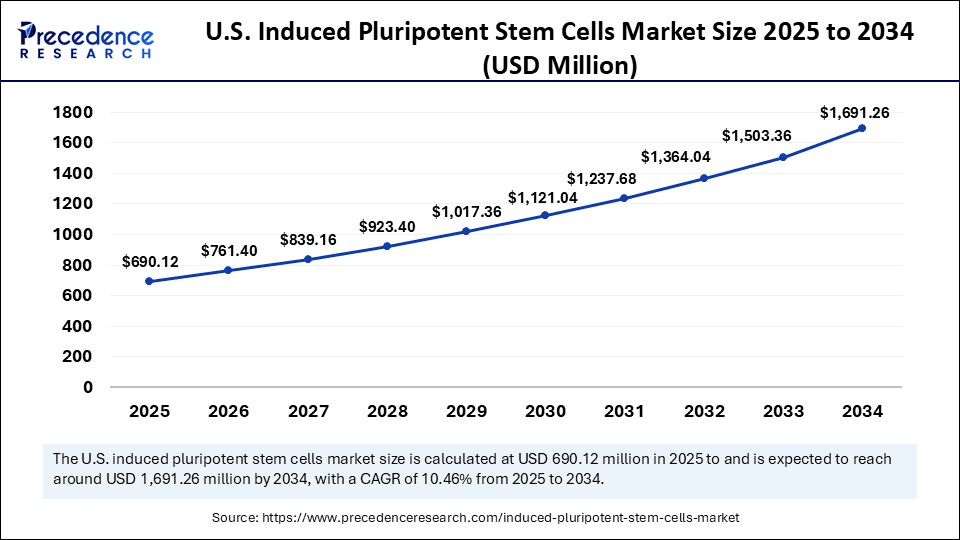
The U.S. induced pluripotent stem cells market size is exhibited at USD 690.12 million in 2025 and is projected to be worth around USD 1,691.26 million by 2034, growing at a CAGR of 10.46% from 2025 to 2034.
Why Did North America Emerge as the Leading Region in the Induced Pluripotent Stem Cells Market in 2024?
North America led the induced pluripotent stem cells market, capturing the largest revenue share in 2024, accounting for an estimated 45% market share, due to the presence of well-developed infrastructure, a high amount of research funding by both the government and the private sectors. Funding The California Institute for Regenerative Medicine (CIRM) and the NIH Stem Cell Program have provided funds for large numbers of focused grants to spur translational iPSC research as well as policymaking regulations. The main centers of academic excellence, such as Harvard Stem Cell Institute, Gladstone Institutes, and Johns Hopkins Institute of Cell Engineering, were innovation-driven by collaborations with biopharmaceutical firms.
It is expected that regulatory clarity on the United States Food and Drug Administration, especially with non-integrating reprogramming technologies and autologous cell therapies, will maintain regional leadership in the short run. The Mayo Clinic Center for Regenerative Medicine initiated a new iPSC-derived chondrocytes clinical program to treat Osteoarthritis as the transition to the broad phenomenon of large-scale therapy. Furthermore, the launch of new solutions by large organizations in this region further facilitates the market in the coming years.
In January 2025, Pluristyx, a leading provider of Good Manufacturing Practices (GMP), cutting-edge, induced pluripotent stem cell (iPSC) technologies, announced the immediate availability of the PSXi013 iPSC line made under GMP. This off-the-shelf, readily available cell line will revolutionize the cell and gene therapy landscape, breaking the mold of how cells are supplied and offering an unprecedented solution for researchers and developers seeking to accelerate clinical translation of their iPSC-based therapies.
(Source: https://pluristyx.com)
North America: U.S. Induced Pluripotent Stem Cells Market Trends
The U.S. has strong government and private funding, and a mature biotech ecosystem. The widespread adoption of iPSCs for disease modelling and drug development, and the expansion of clinical trials for regenerative medicine and cell therapies.
- In May 2025, XellSmart Biopharmaceutical received approval from both the FDA and China's NMPA for a Phase I clinical trial using allogeneic iPSC-derived neural cells for spinal cord injury (SCI), making it the first such trial globally for SCI. (https://www.prnewswire.com)
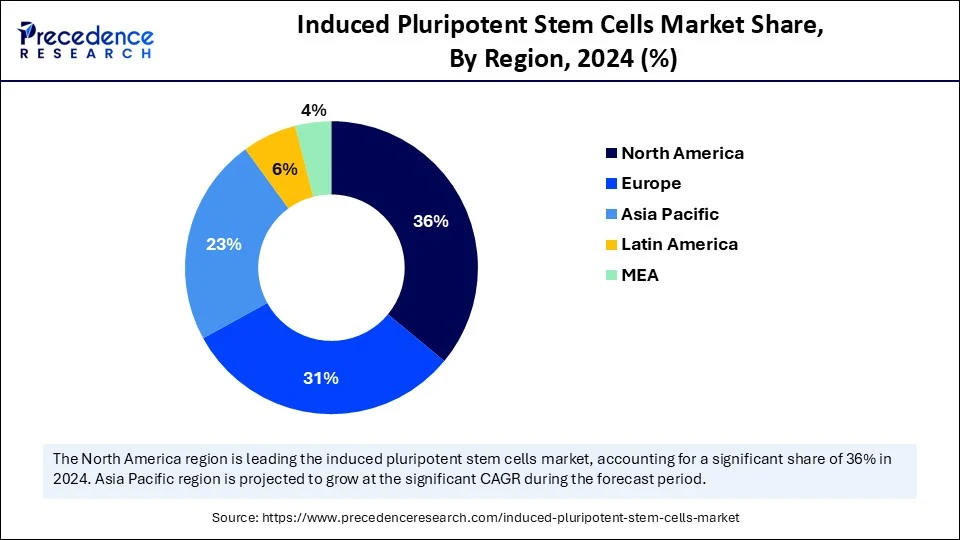
Asia Pacific is anticipated to grow at the fastest rate in the market during the forecast period, owing to the superior government investments growth in the prevalence of chronic diseases that iPSC-based therapies treat. China the National Stem Cell Resource Center developed region-specific iPSC banks to support precision medicine projects in the country. There have also been partnerships, based on the knowledge platform, to scale iPSC reprogramming and differentiation services to enable local clinical trials.
Asia Pacific: China Induced Pluripotent Stem Cells Market Trends
China's iPSC market is expanding rapidly, driven by substantial government funding and a supportive regulatory environment that accelerates clinical translation. A high volume of world-first clinical trials for conditions like spinal cord injury and stroke, and a strong focus on developing scalable, off-the-shelf allogeneic therapies.
- In September 2024, the National Medical Products Administration (NMPA) issued new pilot programs to expand openness in the healthcare sector, allowing foreign-invested companies to participate in human stem cell technology within free trade zones. (https://cms-lawnow.com)
A new and improved regulation framework was proposed in 2024 by the regional governments in South Korea, Australia, and Singapore. They improved the timeline of getting stem cell-based therapy approved, which boosted investment and start-up biotechnology companies. Furthermore, the improvement in enrollment in regional iPSC clinical trials, notably cardiology and neurodegenerative disease trials in Japan, China, and India, is thus expected to lead to momentum in the market.
Value Chain Analysis of the Induced Pluripotent Stem Cells Market
- Research & Development (R&D) and Reprogramming
This initial stage focuses on developing and refining the methods for reprogramming somatic cells (like fibroblasts) into iPSCs, ensuring high efficiency and genetic integrity.
Key Players: Academic and research institutes, NIH, Thermo Fisher Scientific Inc., Applied StemCells Inc., REPROCELL Global. - Cell Culture and Expansion
Once reprogrammed, iPSCs are cultured and expanded in specialized media and bioreactors to produce large, consistent quantities of cells for research or clinical use.
Key Players: FUJIFILM Cellular Dynamics, Inc., Lonza Group AG, Thermo Fisher Scientific Inc., REPROCELL Global, Takara Bio USA, Inc. - Cell Characterization, Banking, and Engineering
Cells are rigorously characterized using techniques like flow cytometry and genetic analysis to confirm pluripotency, purity, and genomic stability.
Key Players: Applied StemCells Inc., FUJIFILM Cellular Dynamics, Inc., REPROCELL Global, Academic and research institutes, Merck KGaA. - End-User Applications and Therapeutics Development
This stage involves the translation of iPSC technology into end-user applications like disease modeling, toxicology screening, and regenerative medicine.
Key Players: Pharmaceutical & biotechnology companies, Fate Therapeutics, Vertex Pharmaceuticals, Astellas Pharma Inc., BlueRock Therapeutics, Axol Bioscience Ltd.
Top Companies in the Induced Pluripotent Stem Cells Market & Their Offerings:
- Allele Biotechnology & Pharmaceuticals: Allele develops and utilizes highly efficient iPSC generation technologies, offering cell lines, media, and contract research services to academic and pharmaceutical clients.
- Axol Bioscience Ltd.: Axol provides iPSC-derived cells, such as neurons, cardiomyocytes, and astrocytes, to researchers for use in drug discovery and disease modeling.
bit.bio (synthetic biology iPSC platform): bit.bio applies synthetic biology to reprogram iPSCs into specific, consistent cell types at scale for research and potential therapeutic use. - BlueRock Therapeutics (Bayer): BlueRock, a subsidiary of Bayer, is a leading player in iPSC-based cell therapy, with a focus on developing treatments for neurodegenerative diseases like Parkinson's. Their contributions involve advancing iPSC therapies through clinical trials and developing scalable manufacturing processes for widespread patient access.
- BrainXell, Inc.: BrainXell specializes in providing iPSC-derived central nervous system cell types, including various types of neurons and glia, for neurodegenerative disease research and drug screening. They contribute to the market by offering high-quality, relevant cell models that facilitate neurological research.
- Cellular Dynamics International (Fujifilm Group): As part of the Fujifilm Group, CDI is a major producer of high-quality, induced pluripotent stem cells and iPSC-derived human cells for drug development and safety testing. They contribute to the market by offering large-scale, consistent cell supply and leading the way in automation and GMP production.
- Century Therapeutics: Century Therapeutics is focused on developing allogeneic iPSC-derived cell therapies for cancer treatment. They contribute to the market by leveraging iPSC technology to create off-the-shelf immunotherapies for widespread patient use.
- Corning Incorporated: Corning provides a wide range of cell culture solutions, including specialized media, surfaces, and vessels essential for the efficient and scalable growth of iPSCs. They contribute to the market by enabling the industrialization and standardization of iPSC manufacturing processes.
- Evotec SE: Evotec utilizes iPSC technology extensively in its drug discovery and development services platform for pharmaceutical clients. They contribute to the market by providing integrated solutions for disease modeling, compound screening, and preclinical testing, accelerating the R&D pipeline.
- Fujifilm Cellular Dynamics, Inc. (FCDI): FCDI is a prominent developer of iPSC and iPSC-derived human cells for use in drug discovery and safety testing. They contribute to the market by supplying highly validated, pure cell types for research and spearheading the move towards automated, large-scale production.
- Lifeline Cell Technology: Lifeline provides cell culture media, supplements, and related reagents tailored for the optimal growth and differentiation of iPSCs. They contribute to the market by supplying essential components for daily research and manufacturing activities.
- Lonza Group AG: Lonza is a leading contract development and manufacturing organization (CDMO) that provides services and media for the production of clinical-grade iPSCs and cell therapies. They contribute to the market by enabling reliable and compliant large-scale manufacturing for biopharma companies.
- Ncardia: Ncardia develops and commercializes iPSC-derived cells and services for drug discovery and safety pharmacology research, particularly focusing on cardiac and neurological models.
- Newcells Biotech: Newcells specializes in using iPSC-derived cell models, particularly for kidney and liver research, to assist in drug development and toxicology testing for pharmaceutical companies. They contribute to the market by offering high-quality, disease-relevant models for complex organ systems.
- Pluricell Biotech: Pluricell is a biotechnology company focused on the development of iPSC-derived cell therapies for degenerative diseases, aiming to move treatments from the lab to the clinic. They contribute to the market by developing novel therapeutic approaches using iPSC technology.
- REPROCELL Inc.: REPROCELL offers a comprehensive range of iPSC-related products and services, including cell lines, media, and contract research services, to support global R&D efforts. They contribute to the market by providing essential tools and services across the iPSC value chain.
- Stemcell Technologies Inc.: Stemcell Technologies provides a wide array of cell culture media, reagents, and instruments necessary for the isolation, expansion, and differentiation of iPSCs. They contribute to the market by supplying high-quality, standardized products for both research and clinical applications.
- Sumitomo Pharma Co., Ltd.: Sumitomo Pharma is actively involved in the development of iPSC-based therapies for various diseases, collaborating with research institutions to translate discoveries into clinical treatments. They contribute to the market by funding and pursuing innovative cell therapy pipelines.
- Takara Bio Inc.: Takara Bio provides a variety of reagents, kits, and services for iPSC generation, culture, and differentiation, including options for clinical research and manufacturing. They contribute to the market by offering tools that ensure high efficiency and compliance with regulatory standards.
- Thermo Fisher Scientific, Inc.: Thermo Fisher is a dominant supplier of tools, reagents, and instruments essential for every stage of iPSC research and manufacturing, from reprogramming to cell analysis.
Induced Pluripotent Stem Cells Market Companies
- Allele Biotechnology & Pharmaceuticals
- Axol Bioscience Ltd.
- bit.bio (synthetic biology iPSC platform)
- BlueRock Therapeutics (Bayer)
- BrainXell, Inc.
- Cellular Dynamics International (Fujifilm Group)
- Century Therapeutics
- Corning Incorporated
- Evotec SE
- Fujifilm Cellular Dynamics, Inc.
- Lifeline Cell Technology
- Lonza Group AG
- Ncardia
- Newcells Biotech
- Pluricell Biotech
- REPROCELL Inc.
- Stemcell Technologies Inc.
- Sumitomo Pharma Co., Ltd.
- Takara Bio Inc.
- Thermo Fisher Scientific, Inc.
Recent Development
- In April 2025, Cellistic, a pioneer in induced pluripotent stem cells (iPSC)-based cell therapies, is launching the Echo-NK platform to enable the scalable manufacturing of allogeneic cell therapies to target multiple diseases. Natural Killer (NK) cells are increasingly leveraged in these therapies for their ability to destroy harmful cancerous cells in their early stages, making them a promising option for the treatment of blood cancers, solid tumors, and autoimmune diseases. (Source: https://www.cellistic.com)
- In April 2025, Applied StemCell submitted a Type II Drug Master File (DMF) to the US Food and Drug Administration for its GMP-grade human induced pluripotent stem cell (hiPSC) line. Fate Therapeutics (CA, USA) received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA for its iPSC-derived CAR T-therapy for systemic lupus erythematosus, and Cellino (MA, USA) and Karis Bio (Seoul, South Korea) announced a strategic partnership to manufacture an iPSC-derived cell therapy for cardiovascular disease. (Source: https://www.regmednet.com)
- In October 2024, Aspen Neuroscience, Inc., a private biotechnology company developing personalized (autologous) cell therapies, announced the expansion of its San Diego footprint near its headquarters in Torrey Pines, with a new 22,000 square foot facility for GMP manufacturing of induced pluripotent stem cell (iPSC)-derived cell therapies.(Source: https://www.prnewswire.com)
- In December 2024, Fate Therapeutics, Inc, a clinical-stage biopharmaceutical company dedicated to bringing a first-in-class pipeline of induced pluripotent stem cell (iPSC)-derived cellular immunotherapies to patients with cancer and autoimmune disorders, today presented new clinical and translational data from the Company's FT819 Phase 1 Autoimmunity study for moderate-to-severe systemic lupus erythematosus (SLE) at the American Society of Hematology (ASH) Annual Meeting being held in San Diego, CA.
(Source: https://ir.fatetherapeutics.com)
Latest Announcements by Industry Leaders
Dr. Chikafumi Yokoyama, CEO of REPROCELL Inc.
- In May 2025, REPROCELL is a global leader in providing products and services to support stem cells for clinical and research use. As part of this, REPROCELL launches StemEdit™ Human iPSC non-HLA class 1 (B2M Homo KO) and StemEdit™ Human iPSC non-HLA class 1/2 (B2M/CIITA Homo double KO) cell lines. These cell lines, though intended for research use only, originate from StemRNA Clinical induced pluripotent stem cells (iPSCs) from a healthy donor utilizing our StemEdit™ gene editing technology. "The release of these new hypoimmune StemEdit™ Human iPSC lines represents a key milestone in enhancing immune research and regenerative medicine. By allowing researchers to investigate and modify immune cell interactions with unmatched precision, these cell lines demonstrate REPROCELL's dedication to supporting scientific progress. We will continue to advance and offer world-class tools to drive innovation in cell therapy and immunology."
(Source: https://www.reprocell.com)
Segments covered in the Report
By Application
- Drug Discovery & Toxicology Testing
- High-throughput drug screening
- Hepatotoxicity and cardiotoxicity testing
- Disease Modeling
- Neurological diseases (ALS, Parkinson's, Alzheimer's)
- Cardiovascular and metabolic diseases
- Regenerative Medicine
- Cell-based therapies (retinal, cardiac, hematopoietic)
- Stem Cell Banking
- Academic Research
- Gene Editing Research (iPSC + CRISPR/Cas9)
By Cell Type / Lineage
- Hematopoietic Cells
- Cardiomyocytes
- Neural Stem Cells
- Hepatocytes
- Retinal Pigment Epithelium (RPE)
- Pancreatic Beta Cells
- Chondrocytes & Osteoblasts
- Others (T cells, epithelial cells, etc.)
By Product & Service
- iPSC-Derived Cells
- iPSC Reprogramming Kits & Culture Media
- iPSC Lines & Custom Cell Engineering Services
- CRISPR & Genetic Modification Tools
- Stem Cell Banking Services
- Assay Kits for Differentiation, QC, and Validation
- Instruments & Bioreactors
By End User
- Pharmaceutical & Biotech Companies
- Academic & Research Institutions
- CROs & CDMOs
- Cell Therapy Companies
- Government & Non-Profit Organizations
By Technology
- Integrating Vectors (e.g., Retroviruses)
- Non-Integrating Methods
- Sendai virus
- Episomal vectors
- mRNA & Protein-based reprogramming
- CRISPR-Cas9 & Gene Editing
- Automated iPSC Production Platforms
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East &Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content