Biopharma must address the unique health challenges women face beyond reproduction. Tackling these gaps can lead to improved treatments and healthier lives for everyone.
Almost 50% of the world’s population and 80% of individuals in fields like immunology are women. Despite this, therapies are often created without specific considerations for female patients. Researchers frequently narrow their perspective on "women's health" to reproductive organs, perceive women as "smaller men" when considering other conditions, and neglect essential biological differences like cellular sex (each body cell has a sex), hormonal influences, and genetic components. Tackling these disparities is not merely about fairness it leads to more accurate and effective healthcare for all.
Women’s health is frequently reduced to just sexual and reproductive health (SRH), which significantly undervalues the overall health challenges faced by women. Studies indicate that SRH along with maternal, newborn, and child health (MNCH) represent around 5 percent of the overall health burden faced by women, though this is likely an underrepresentation. Approximately 56 percent of the burden arises from health issues that are either more widespread or present differently in women. The other 43 percent arises from conditions that do not impact women more or differently based on existing evidence. The disparity in women's health amounts to 75 million lost years of life annually due to inadequate health or premature mortality, translating to seven days lost per woman each year. Bridging the gap could create an effect comparable to 137 million women obtaining full-time jobs by 2040.
The biopharmaceutical sector has a distinct chance to address the health disparity through investments in women’s health. Pharmaceutical companies can promote innovation for women’s health issues like endometriosis, enhance results and minimize risks for common conditions such as heart disease, and explore high-growth markets to boost short-term growth.
As funding from both public and private sectors for women’s health increases, the industry is strongly positioned to provide fair, customized healthcare solutions by incorporating sex-specific approaches from the beginning. In 2019, a total of $122 billion was invested in 3,225 biopharma transactions in the U.S. and Europe, as reported by SVB. Just $1.3 billion was invested in women's health biopharma firms through 60 deals.
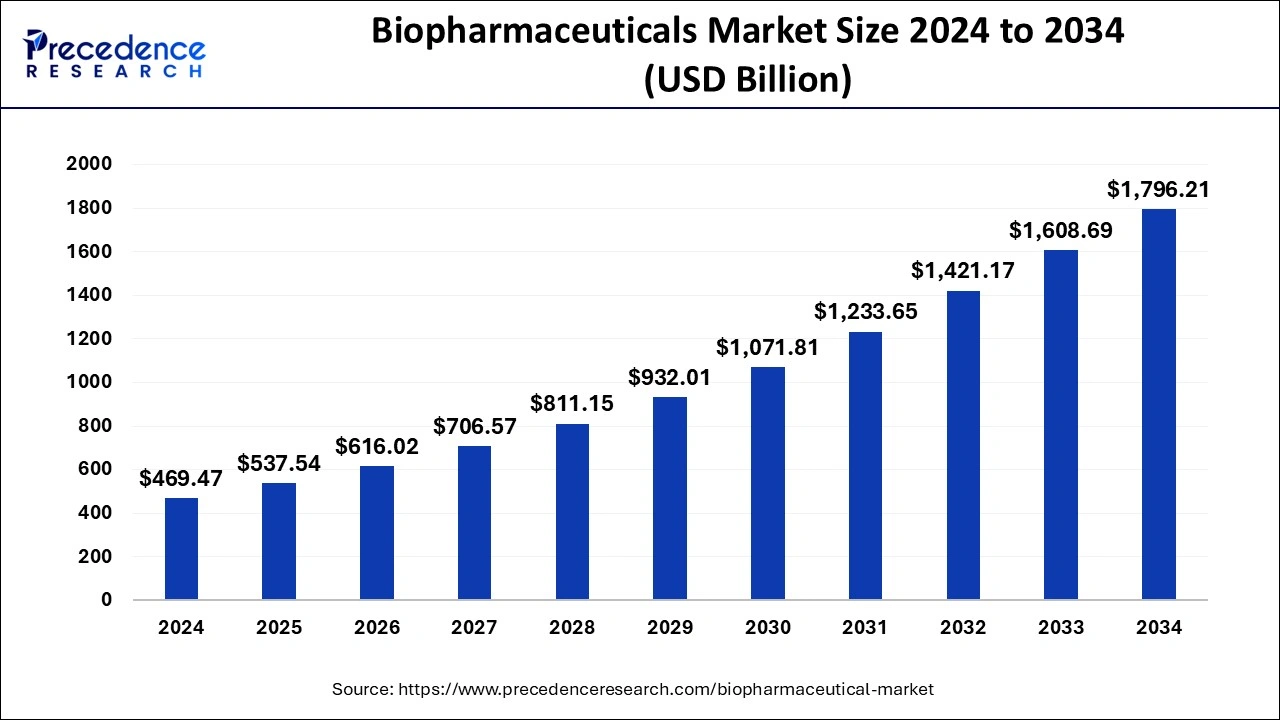
Biopharmaceuticals Market Growth Accelerates: Key Upside for Women-Centric Therapies
According to Precedence Research, the global biopharmaceuticals market size reached USD 389.1 billion in 2023 and is projected to hit USD 856.1 billion by 2033, growing at a CAGR of 8.1% from 2024 to 2033. The sector’s rapid expansion driven by biologics, immunotherapies, and personalized medicine highlights a significant opportunity for pharma companies to integrate women-focused R&D and close long-standing sex-based health gaps.
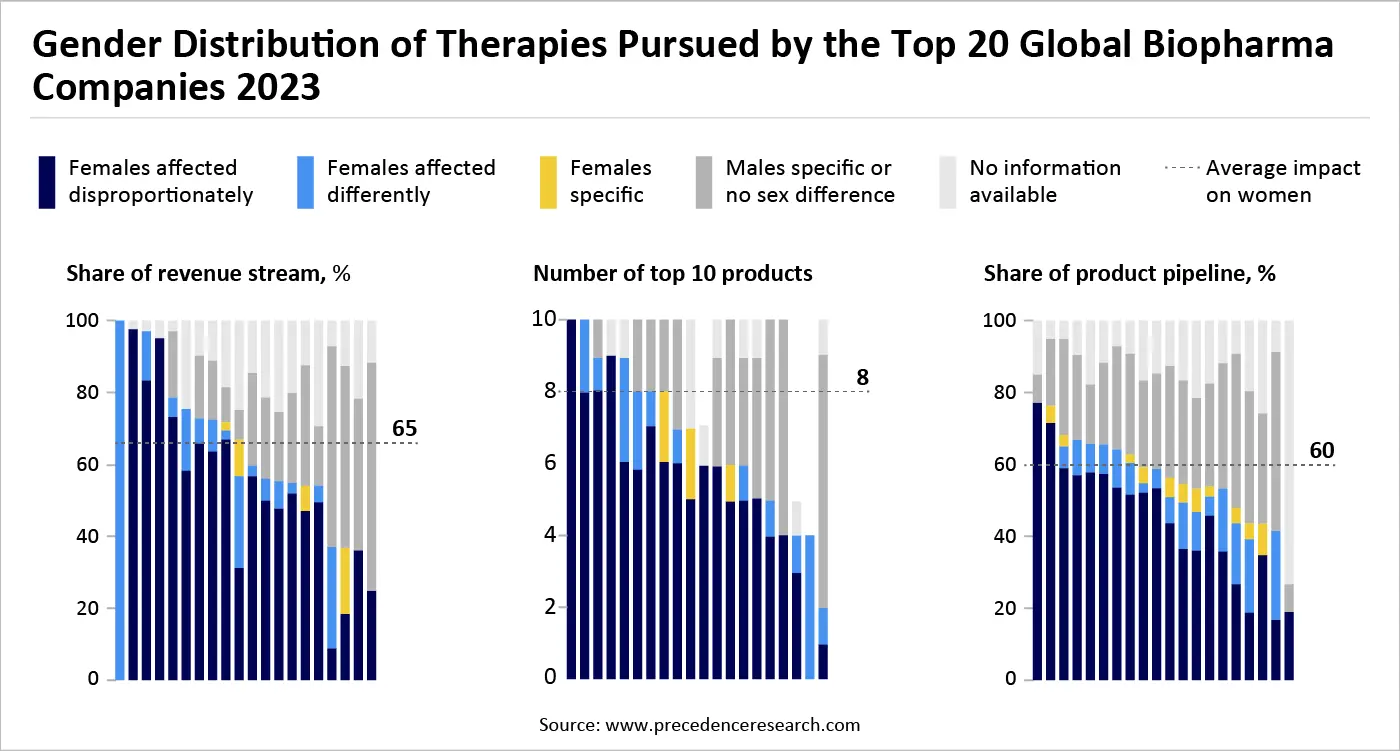
How Biopharma Giants are Already Dominating Women's Health
In addition to venturing into fields with high unmet needs for women, pharma companies can also bridge the health gap by acknowledging and enhancing the significant role women’s health currently occupies in their portfolios. Most of the leading 20 pharmaceutical firms obtain over 60 percent of their revenue from therapies for conditions that uniquely, differently, or disproportionately impact women (Exhibit 1). A significant share of earnings for 16 of these companies is generated from therapies for conditions like autoimmune diseases, mental health issues, osteoporosis, cardiovascular illnesses, and some cancers. (Exhibit 1) Major biopharma companies invest in women's health both through dedicated product portfolios for female-specific conditions (e.g., contraception, menopause, certain cancers) and by developing treatments for general conditions that disproportionately affect women (e.g., autoimmune diseases, osteoporosis, cardiovascular disease).
Here is a table detailing how major biopharma companies have invested in women's health:
|
Company |
Investment Area |
Specific Products / Initiatives |
|
Organon |
Contraception, fertility, established medicines, global access |
Nexplanon (contraceptive implant), NuvaRing (vaginal ring), Follistim (ovulation stimulation), Her Promise Access Initiative (global contraception access) |
|
Pfizer |
Menopause, osteoporosis, breast cancer |
Premarin/Prempro (hormone replacement therapy), Ibrance (breast cancer treatment), funding for companies in women's health advancements |
|
Bayer |
Women's healthcare pipeline expansion, digital health |
Acquisition of KaNDy Therapeutics for an $875 million drug pipeline expansion; digital interventions like 'Bayer For Women' platform and 'Bare your Pain' app |
|
Roche |
Oncology, diagnostics, global access |
Focus on breast, ovarian, and cervical cancers; Global Access Program for cervical cancer diagnostics in Africa |
|
AbbVie |
Endometriosis, uterine fibroids |
Synarel (nasal spray for endometriosis), Orillissa (pain management for endometriosis), Lupron Depot (for fibroids and endometriosis) |
|
Eli Lilly |
Oncology, reproductive health, mental health, bone density |
Verzenio (breast cancer), Prozac (premenstrual dysphoric disorder), Forteo (osteoporosis in postmenopausal women) |
|
AstraZeneca |
Oncology |
Nolvadex, Zoladex (breast cancer treatments), Lynparza (ovarian and breast cancer) |
|
Novartis |
Oncology, osteoporosis, cardiovascular health |
Femara, Afinitor (breast cancer), Reclast (osteoporosis); heart failure drug Entresto found to be particularly effective for women |
Why Sex-Specific Research is the Future of Healthcare
Growing evidence regarding disease differences based on sex underscores the necessity for increased focused research and development for treatment options. These differences encompass the active involvement of the second X chromosome in females, especially regarding immune responses, and biological variations between women and men in fat distribution and metabolism that influence drug effectiveness and safety in cardiometabolic therapies. The pharmaceutical sector is at a pivotal moment where recognizing sex-specific differences could lower clinical trial risks and enhance health results in various populations. With the progress of research capabilities, there is an increasing opportunity to create more precise, effective therapies that consider biological differences in sex. Considering sex differences allows drug developers to create treatments that are safer and more effective. Not considering sex-specific factors can result in a medication being ineffective or potentially harmful for one gender.
In 2013, the US Food and Drug Administration (FDA) took action to adjust the dosage of sleep medication for women, as the suggested dose based on male physiology affected alertness, increasing the risk of accidents while driving to work the following morning. Moreover, tests indicated that women had elevated morning blood concentrations of the drug compared to men, emphasizing the necessity for dosage modifications based on sex. Another example of a sleep aid is the medication Ambien. From 1997 to 2000, eight out of the 10 drugs withdrawn from the market for different reasons were found to have higher risks for women compared to men. Significantly, in 2013, the FDA cut the recommended dose of Ambien for women by 50%, 21 years after it was initially approved.
Recent notable instances highlight the significance of comprehending sex-related disparities in medical therapies. Novartis found that its heart failure medication ENTRESTO, which was introduced in 2015, had notable effectiveness among women, who are twice as likely as men to experience heart failure with preserved ejection fraction (HFpEF). Following clinical trials on specific subgroups, a 2019 Phase III study showed that the medication decreased hospital durations for women by 33 percent. This discovery resulted in broader FDA approval, allowing Entresto to access over two million more patients.
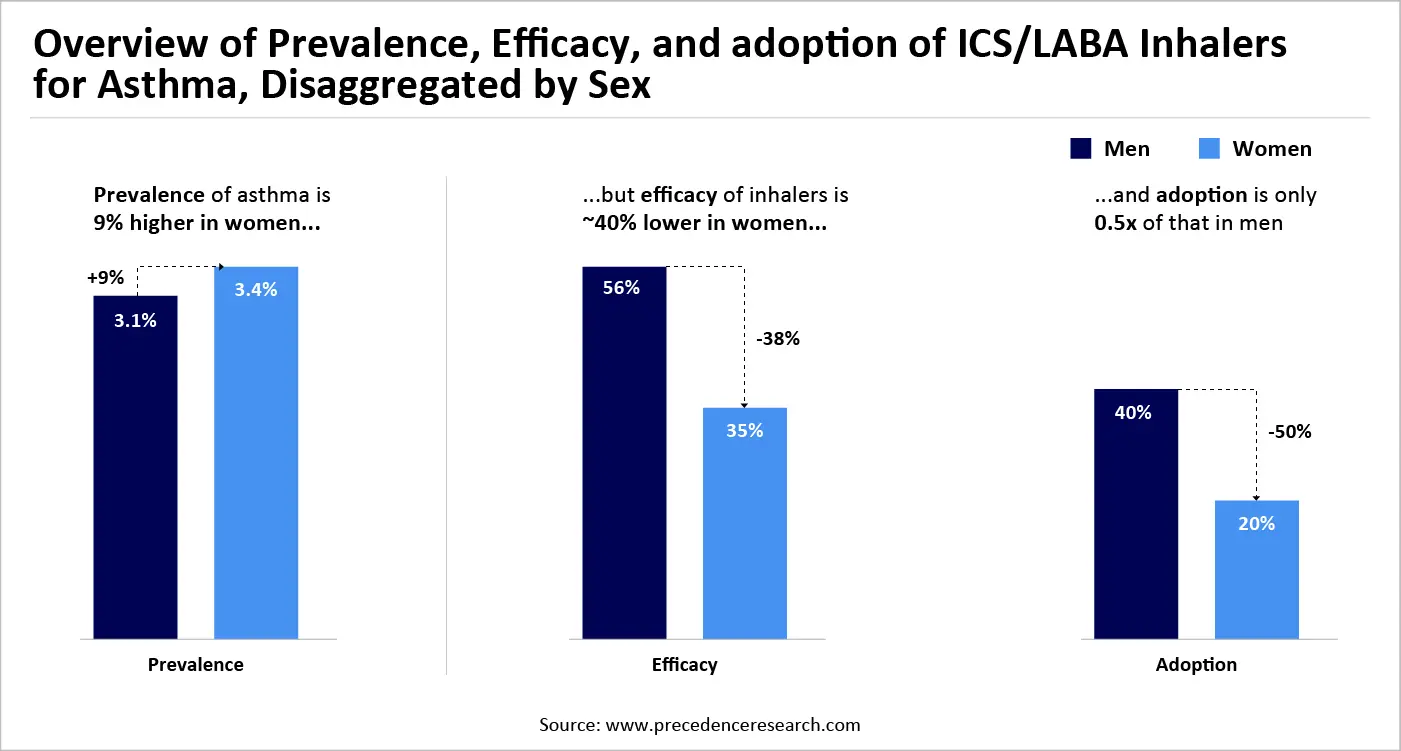
Narrowing the sex gap can significantly influence treatment uptake and effectiveness for women. For example, asthma occurs more frequently in women, but they show reduced treatment effectiveness and lower rates of adherence. Inhaled corticosteroid (ICS)/long-acting beta agonist (LABA) inhalers show around 40 percent reduced effectiveness in women,10 and their usage rates are only 50 percent of those in men,11 partially because of hormonal variations and access challenges. Obtaining gender equality in asthma management may lead to a 27 to 35 percent rise in the number of women receiving effective treatment, benefiting an extra 16 million female patients and averting around 1.6 million disability-adjusted life years (DALYs).
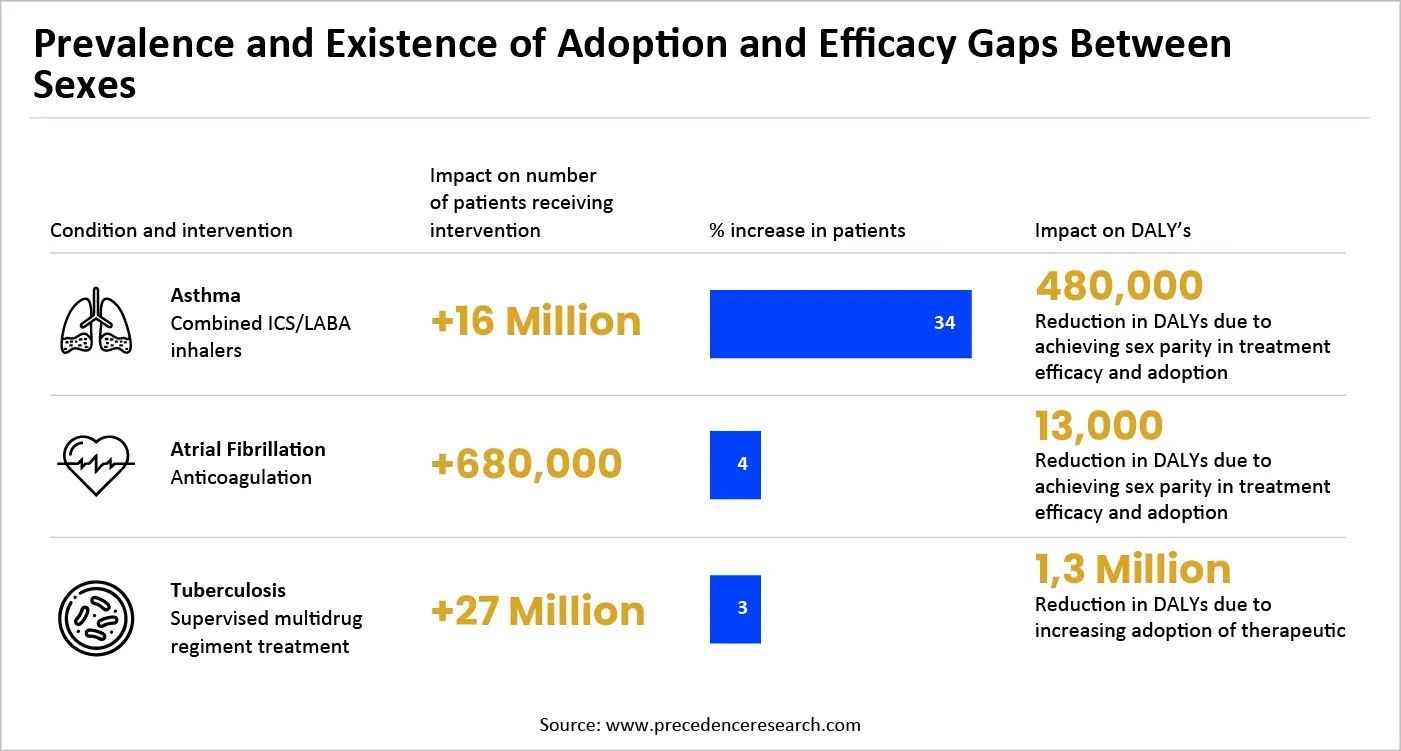
 Comparable advantages can be obtained in different conditions (Exhibit 3). Tackling sex-related adoption disparities in atrial fibrillation and tuberculosis management could together avert 1.4 million DALYs. These statistics highlight the significant health effects of tackling sex disparities throughout the pharmaceutical value chain.
Comparable advantages can be obtained in different conditions (Exhibit 3). Tackling sex-related adoption disparities in atrial fibrillation and tuberculosis management could together avert 1.4 million DALYs. These statistics highlight the significant health effects of tackling sex disparities throughout the pharmaceutical value chain.
By adopting a vigilance-based strategy in clinical trials and a knowledge growth mentality focused on women’s health, researchers can address sex-based differences more effectively, ensuring equitable, personalized care that improves outcomes for all patients. By closing the sex gap in medical research, we can create a more inclusive healthcare system that takes into account the unique biological needs of all individuals, ultimately leading to better outcomes and reducing health disparities. This shift is not only necessary for advancing women’s health, but also for ensuring that healthcare interventions are effective, safe, and equitable for everyone.
New regulations are also targeting women’s health. For example, the US Center for Devices and Radiological Health now require sex-specific testing for medical devices, recognizing the impact of physiological and anatomical differences on safety and efficacy. Such new standards foster accountability, transparency, and innovation, ensuring safer, more effective interventions.
The table below outlines key regulatory and policy trends impacting women's health regarding diagnosis, treatment, and medical devices.
|
Category |
Region |
Regulation/Policy |
Key Provisions Targeting Women's Health (or general health impact) |
|
Diagnosis & Screening |
EU |
In Vitro Diagnostic Medical Devices Regulation (IVDR) (EU) 2017/746 |
Stricter guidelines for IVDs (e.g., diagnostic tests), including enhanced clinical data requirements and post-market surveillance. This impacts diagnostic tools for conditions like cervical cancer (e.g., HPV tests). |
|
US (Policy Trend) |
FDA approach to Diagnostics |
The FDA has cleared new diagnostics for menopause status (e.g., PicoAMH Elisa test) to assess a woman's final menstrual period status, encouraging preventative care discussions. |
|
|
Global (WHO) |
Guidelines on the clinical management of arboviral diseases |
Provides updated guidelines for diseases like Zika, which pose significant risks during pregnancy. |
|
|
Treatment & Medication |
US (Policy Trend) |
Post-Dobbs Decision Landscape |
The Supreme Court decision in 2022 removed the federal right to abortion, leading to highly variable access to abortion care and related reproductive health services across different states, where regulations are now set at the state level. |
|
Global (WHO) |
Abortion Care Guideline, 2nd ed. (due Sep 2025) |
A comprehensive set of all WHO recommendations and best practice statements related to abortion care, aiming to provide evidence-based global standards. |
How Pharmaceutical Companies Can Address Sex-Based Health Disparities
Our analysis suggests, pharmaceutical companies can play a crucial role in narrowing the health disparity between men and women by adopting a fresh approach to value creation. They must implement clear strategies to tackle sex-based disparities in R&D, commercial, and medical affairs. By reevaluating R&D methods, improving the patient experience, and fine-tuning commercialization tactics, companies can integrate a women’s health perspective into their current operations and create substantial value while elevating health results for women.
Data generation and analysis, which serve a pivotal role in both R&D and commercialization operations, can establish a basis for informed decision-making and impactful women’s health initiatives. For instance, information regarding drug adoption rates, effectiveness, and safety results broken down by both sex and gender is limited. There is sparse information regarding the impact of medical treatments on diverse populations, especially the LGBTQ+ community, including individuals receiving hormonal therapy, who may face distinct effects and need tailored care.
The Role of R&D in Increasing Drug Efficacy and Patient Safety
R&D organizations can aid in narrowing the women’s health gap by incorporating a sex-specific strategy at each phase of research and development. Integrating these findings can improve the efficacy and safety of therapies for every patient, thereby promoting the objective of genuinely personalized medicine.
Five levers can assist R&D organizations in facilitating this change:
- Perform preclinical research specific to sex. Female cell lines should be utilized for in-vitro testing. Researchers need to go beyond typical male animal models to gain a deeper understanding of female responses, particularly in sex-specific conditions, while collaborating with governmental research agencies and academic institutions to create more representative models.
- Boost fundamental research on female-specific ailments that have substantial unmet needs and considerable market opportunities. Conditions like endometriosis and polycystic ovary syndrome impact millions of women, but they continue to receive insufficient funding and research attention. Endometriosis, for instance, impacts one in ten women worldwide and signifies a market opportunity of $180 billion to $250 billion, comparable to major investment sectors like immunology and diabetes. By collaborating to focus additional research on these conditions, R&D organizations can meet significant unmet needs, develop tailored treatments for women, and realize significant economic benefits from these high-potential markets.
- Create an asset strategy that focuses on women’s health. R&D organizations can customize their development strategy and target product profile (TPP) according to how conditions impact women differently from men. This entails considering the comparative frequency of men and women, the intended outcome, and the safety profile, factoring in sex-specific limits.
- Develop studies that incorporate and consider sex-specific variations while ensuring a suitable balance of participation by sex. Protocols must be crafted to account for potentially varied inclusion and exclusion standards according to different disease presentations in males compared to females. They must also consider sex-specific variations and incorporate endpoints to evaluate sex-specific impacts, like alterations in hormonal balance or the menstrual cycle. Women frequently face underrepresentation in trials due to obstacles like transportation issues, childcare responsibilities, and inflexible schedules. Trial managers might provide adaptable options like remote oversight and logistical assistance to enhance accessibility for women and to speed up recruitment and completion periods. They can likewise partner with women's health organizations and medical facilities to recognize and tackle obstacles faced by women. They can subsequently improve recruitment and retention methods to make sure that trials represent actual female populations and expand outreach to various groups. They might collaborate with digital health firms to gather important, real-world insights specific to sex from telemedicine and wearable data.
- Finalization and documentation clinical trial reports and analyses are essential domains where sex differences must be taken into account. Disaggregated trial data allows researchers and healthcare providers to evaluate sex-based treatment results and establish beneficial feedback mechanisms for ongoing enhancement in meeting sex-specific health requirements.
Key Examples of Company R&D Strategies Include:
Organon serves as a prominent illustration of a company focused on a primary mission for women's health.
R&D Strategy: Organon is consistently developing an R&D portfolio addressing diverse women's health concerns, which for 2024 emphasized:
- Postpartum hemorrhage (PPH): A major factor in maternal mortality globally, an aspect traditionally underrepresented in R&D.
- Endometriosis-related pain: A prevalent issue impacting 1 in 10 women.
- Contraception without hormones, on request: Offering non-hormonal choices for women who favor them.
Access Initiatives: The organization operates a "Her Promise Access Initiative" to enhance the availability of contraception and education in economically disadvantaged nations.
Collaborations: Organon obtained the worldwide rights for ebopiprant, a trial medication aimed at preventing preterm birth, from ObsEva.
UCB: Customizing Medications for Pregnancy
UCB emphasized collecting particular information about its chronic inflammatory disease treatment, Cimzia, to guarantee its safety for an important group of women.
R&D Approach: UCB performed research that specifically monitored the extent to which its medication Cimzia transferred into the placenta and breast milk, discovering the transfer was negligible.
Result: This resulted in an FDA label extension in 2024, permitting pregnant women with chronic inflammatory conditions to maintain treatment, a notable progress for the 17% of the qualifying patient group that consists of women in their childbearing years.
Leveraging Commercialization to Increase Awareness and Understanding of Women's Health
Pharmaceutical companies can achieve better results right away by integrating a women’s health viewpoint into their existing offerings. They can achieve this by enhancing their comprehension of a woman's patient experience and incorporating that insight into their marketing strategies. This method can aid in bridging gaps related to sex and gender, especially in diagnosis, treatment escalation, and adherence. Essential tactics comprise the subsequent:
- Targeted marketing strategies. Marketing teams can create strategies specifically designed for women, particularly in areas where awareness is lacking, like autoimmune disorders and heart diseases. Emphasizing the impact of these conditions on women can enhance engagement, shorten diagnostic delays, and assist women in prioritizing their well-being.
- Focused awareness and educational initiatives. Medical education today offers insufficient exposure to information regarding sex-based differences, resulting in many providers being inadequately informed about how these differences influence diagnosis and treatment. Commercial teams can bridge this knowledge gap by providing educational materials and programs focused on sex-specific symptoms and management techniques for conditions that impact women uniquely or excessively, such as cardiovascular disease, autoimmune disorders, and mental health issues. Training sessions, webinars, and workshops can assist providers in understanding how conditions present variably in women, leading to more precise and swift diagnoses. For instance, increased understanding of the various symptoms women encounters during heart attacks like nausea, fatigue, and back pain, instead of the more widely acknowledged chest pain can result in earlier detection and prompt care, which is vital, considering that women are over twice as likely to die post-heart attack compared to men.
- Tailored patient assistance initiatives. Business leaders could create patient support programs that acknowledge women’s specific healthcare requirements and that provide methods for assisting them in their health journeys. They may collaborate with digital health platforms to create content and resources that tackle health-related challenges more common for women and encourage adherence to treatment. Data from these digital platforms (including wearables and telemedicine interactions) can provide valuable real-world evidence (RWE) for tracking metrics pertinent to women’s health, such as hormone levels and variations associated with the menstrual cycle. RWE is especially useful for enhancing drug therapies after they are launched, enabling pharmaceutical companies to consistently modify strategies using data-informed insights.
Integrating Sex-Based Perspectives into Pharma's Core Mission and Strategy
- Incorporating sex-based viewpoints into the mission of pharma can lead to significant improvements in women's health by emphasizing change management, beginning with leadership. Leaders should proactively advocate for a strategy that recognizes sex-related differences, not only in patient treatment but throughout all organizational operations. This dedication conveys a message across the organization that promoting women’s health is a key goal ingrained in the company’s mission.
- To achieve enduring change, businesses need to incorporate a gender-based perspective into their culture and their decision-making processes. This starts with extensive training and awareness initiatives that enable employees in R&D, commercial, and patient-facing teams to recognize the significance of sex-based factors.
- Accountability measures are also essential for maintaining progress. Leaders must set specific metrics to monitor the implementation of sex-based strategies and ensure teams are responsible for achieving these goals. Companies can also pledge to report transparently on these initiatives, strengthening their dedication to health equity and fostering trust with stakeholders.
- Ultimately, integrating sex-based factors into fundamental governance processes is crucial for achieving systematic change. This entails incorporating sex-related factors as a regular agenda item for governance groups managing essential deliverables, like asset teams evaluating target product profiles or clinical teams planning trials.
About the Authors

Aditi Shivarkar
Aditi, Vice President at Precedence Research, brings over 15 years of expertise at the intersection of technology, innovation, and strategic market intelligence. A visionary leader, she excels in transforming complex data into actionable insights that empower businesses to thrive in dynamic markets. Her leadership combines analytical precision with forward-thinking strategy, driving measurable growth, competitive advantage, and lasting impact across industries.

Aman Singh
Aman Singh with over 13 years of progressive expertise at the intersection of technology, innovation, and strategic market intelligence, Aman Singh stands as a leading authority in global research and consulting. Renowned for his ability to decode complex technological transformations, he provides forward-looking insights that drive strategic decision-making. At Precedence Research, Aman leads a global team of analysts, fostering a culture of research excellence, analytical precision, and visionary thinking.

Piyush Pawar
Piyush Pawar brings over a decade of experience as Senior Manager, Sales & Business Growth, acting as the essential liaison between clients and our research authors. He translates sophisticated insights into practical strategies, ensuring client objectives are met with precision. Piyush’s expertise in market dynamics, relationship management, and strategic execution enables organizations to leverage intelligence effectively, achieving operational excellence, innovation, and sustained growth.





 Request Consultation
Request Consultation

