What is the Laboratory Products and Outsourcing Services Market Size?
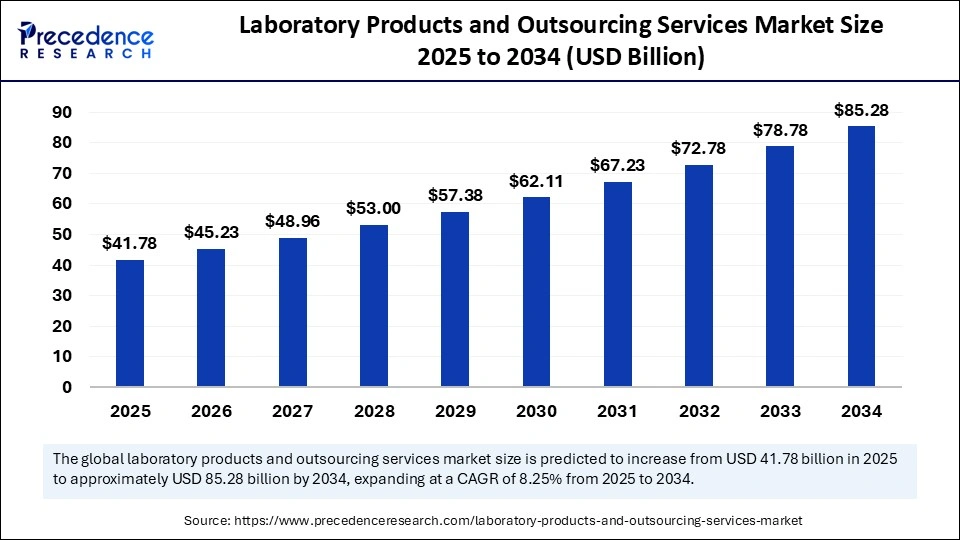
The global laboratory products and outsourcing services market size is valued at USD 41.78 billion in 2025 and is predicted to increase from USD 45.23 billion in 2026 to approximately USD 85.28 billion by 2034, expanding at a CAGR of 8.25% from 2025 to 2034. The rising occurrence of intricate diseases and the need for improved diagnostics are fueling expansion in the market. The increasing use of personalized medicine, non-invasive technologies, and outsourcing for cost-effectiveness and regulatory adherence also drives market growth.
Laboratory Products and Outsourcing Services Market Key Takeaways
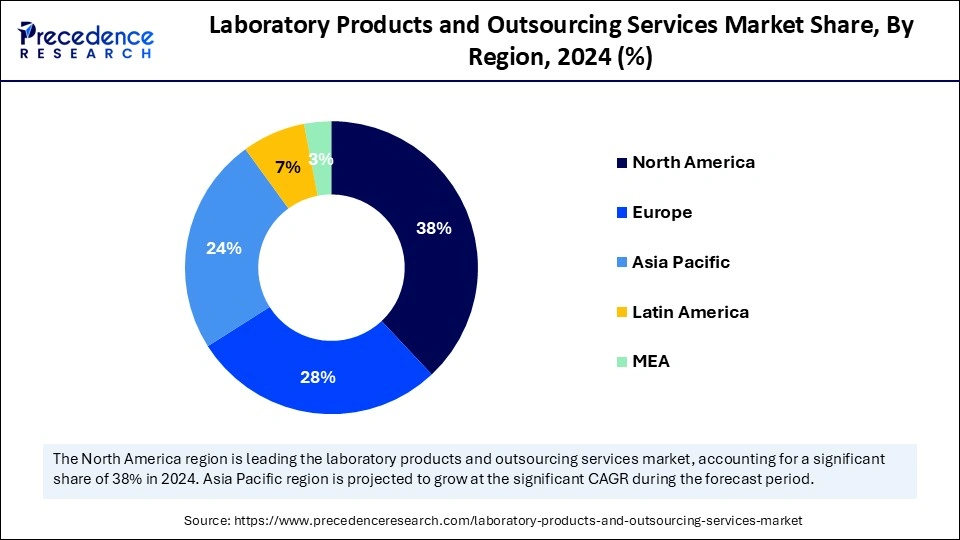
- North America dominated the global market with the largest market share of 38% in 2024.
- The Asia Pacific market is anticipated to grow at the fastest CAGR during the forecast period.
- Europe plays an important role in the growth of the global market.
- By type, the products segment contributed the biggest market share of 63% in 2024.
- By type, the services segment is expected to witness the fastest CAGR of growth during the forecast period.
- By technology, the molecular diagnostics segment held a dominant presence in the market in 2024.
- By technology, the immunoassays segment is projected to expand rapidly in the coming years.
- By end-use, the medical device segment held the major market share in 2024.
- By end-use, the pharmaceutical and biotech companies segment is projected to grow with the fastest CAGR in the forecast period.
Artificial Intelligence Driving Precision and Efficiency in Research for Laboratory Products
Artificial intelligence is transforming the laboratory products and outsourcing services market by improving efficiency and precision via automation and data evaluation. It enhances the handling of extensive datasets, increases reproducibility, and reduces human mistakes. Important uses involve automated data evaluation, forecasting models, and robotics integration, which enhance workflows such as sample management and assay processing. AI enhances the analysis of experimental results, shortens turnaround times, and optimizes resource use, especially in drug discovery and clinical trials, thereby boosting overall productivity.
- In late 2024, Dash Bio, a biotech startup, launched AI-driven robotic solutions for clinical trial sample analysis, decreasing turnaround times and improving drug development efficiency, showcasing the future of outsourcing in the healthcare and biotechnology industries.
Empowering Research: How Laboratory Products and Outsourcing Services Are Transforming Healthcare Biotechnology
The market for laboratory products and outsourcing services consists of external organizations that offer crucial services and products for research, development, and testing, particularly in the fields of healthcare and biotechnology. This market has grown because of the rising intricacy of research and a need for affordable options.
This market encompasses a diverse array of products and services utilized in various sectors, such as pharmaceuticals, healthcare, and academics, focused on improving efficiency, cost-effectiveness, and specialization. Market services involve delegating tasks such as clinical trials and testing to specialized firms, enabling organizations to concentrate on primary activities while leveraging external expertise.
Laboratory Products and Outsourcing Services Market Growth Factors
- Global expansion of clinical trials: The demand for preclinical testing services is growing notably because of the rising number of clinical trials globally. Contract Research Organizations are growing because of cost reductions and regulatory support, signaling a move towards customized medicine and novel therapies. This expansion is driven by the availability of skilled professionals and the demand for sophisticated laboratory services.
- Enhanced research and development funding: Governments and private sectors are boosting Research and Development funding in biotechnology, pharmaceuticals, and personalized medicine, acquiring advanced laboratory products and outsourcing specialized services to enhance results.
- Rising need for specialized services: The increasing complexity of scientific research demands specialized services, such as niche testing and molecular diagnostics tools, to deliver high-quality analysis and testing for customized treatments.
- Technological advancements: Artificial intelligence and automation are transforming laboratory operations by boosting data analysis, optimizing workflows, and increasing precision. This has boosted the demand for outsourcing, as intelligent tools with real-time monitoring and data analysis features enhance efficiency.
- Cost and time efficiency: Laboratory products and outsourcing services enhance cost-effectiveness, particularly for biotech and pharmaceutical firms. It lowers internal operational expenses and speeds up the time to launch new products, aiding smaller businesses and startups by providing access to advanced technology and knowledge.
Market Outlook
- Industry Growth Offerings- The market is growing due to rising R&D investments, increasing demand for specialized testing, technological advancements, cost-effective solutions, and the ability to streamline research processes, enabling companies to focus on core development activities.
- Global Expansion- Global expansion of the market is driven by rising pharmaceutical and biotech R&D worldwide, increasing demand for specialized testing, cost-efficiency, regulatory compliance needs, and adoption of advanced technologies across healthcare, academics, and industrial sectors.
- Startup ecosystem- The startup ecosystem for laboratory products and outsourcing services is thriving due to growing investment in biotech and healthcare innovation, emergence of specialized testing solutions, digital lab technologies, and opportunities to offer agile, cost-effective, and scalable R&D services globally.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 85.28 Billion |
| Market Size in 2025 | USD 41.78 Billion |
| Market Size in 2026 | USD 45.23 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.25% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Technology, End Use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Market Dynamics
Drivers
Need for cost-efficiency and operational adaptability
The laboratory products and outsourcing services market is propelled by cost-effectiveness and operational adaptability, enabling organizations to enhance resource utilization, minimize capital expenditures, and adjust to market fluctuations. Outsourcing tasks such as analytical testing, clinical trials, and regulatory compliance reduces operational expenses, speeds up procedures such as drug development, and enables companies to concentrate on their primary strengths.
- In February 2025, Amgen, a pharmaceutical firm based in the U.S., allocated USD 200 million to a technology center in Hyderabad, utilizing skilled workforce and cost benefits of India with an emphasis on artificial intelligence and data science for developing drugs. The center now has 300 staff members, intending to grow to 2,000 by the close of 2025.
Restraint
Data breaches and the loss of intellectual property
A major restraint in the laboratory products and outsourcing services market is the potential for data breaches and the loss of intellectual property. As companies outsource, they provide sensitive information to external service providers, which raises concerns regarding cybersecurity. Sending sensitive information is frequently necessary, putting organizations at risk of cyberattacks. A report from the U.S. Department of Homeland Security showed that almost 60% of organizations experienced cybersecurity incidents connected to outsourcing in the previous year.
Opportunity
Adoption of personalized medicine and companion diagnostics
The laboratory products and outsourcing services market is experiencing an increase in the adoption of personalized medicine and companion diagnostics. This trend is driven by advancements in genomics and biotechnology, requiring laboratory services to facilitate the creation and application of personalized therapies, ensuring accurate treatment targeting according to the unique genetic composition of an individual.
- In August 2024, the FDA sanctioned the TruSight™ Oncology Comprehensive test of Illumina, which analyzes more than 500 genes in solid tumors to detect particular genetic mutations, emphasizing the role of laboratory services in tailored treatments.
Type Insights
The products segment dominated the laboratory products and outsourcing services market with the largest share in 2024, addressing various laboratory requirements that necessitate specialized equipment, consumables, reagents, and software solutions. This segmentation enables businesses to customize their products to address needs in areas such as clinical diagnostics, drug discovery, and environmental testing. The rising demand for specialized equipment in areas such as biotechnology, clinical diagnostics, and pharmaceuticals is fueling the expansion of the market.
- In December 2024, Thermo Fisher Scientific collaborated with AstraZeneca to supply advanced laboratory tools for tailored therapies, emphasizing genomic profiling and biomarker identification, showcasing advanced NGS technologies of Thermo Fisher.
The services segment is expected to witness the fastest rate of growth during the predicted timeframe, because of the rising intricacy of drug development procedures and rigorous regulatory standards. Pharmaceutical firms encounter difficulties in introducing new medications, as around ninety percent of drug candidates fail in clinical trials. The increased demand for specialized knowledge in bioanalytical testing services, encompassing sub-categories such as Absorption, Distribution, Metabolism and Excretion (ADME), Pharmacokinetics (PK), Pharmacodynamics (PD), bioavailability and bioequivalence testing, is fueling expansion in the service segment. The intricacies of drug development, stringent regulatory requirements, and elevated drug failure rates fuel the market, underscoring the demand for careful testing services to reduce risks linked to drug development.
Technology Insights
The molecular diagnostics segment held a dominant presence in the market in 2024 because of its vital importance in the early detection, diagnosis, and monitoring of diseases, particularly for infectious diseases and cancers. Improvements in molecular diagnostic technologies such as PCR, DNA sequencing, and next-generation sequencing have resulted in precise and swift outcomes, crucial for effective management and treatment approaches for patients. The increasing need for personalized medicine requires accurate diagnostic tools to determine individual genetic profiles, thereby boosting the use of molecular diagnostics. The worldwide increase in infectious diseases has intensified the demand for swift and dependable diagnostic techniques, positioning molecular diagnostics as an essential component of the market.
- In October 2024, Bio-Rad Laboratories saw a 5.6% growth in sales for Q3 2024, totaling USD 388.8 million, mainly driven by heightened demand for diagnostic products and a rising dependence on molecular diagnostics in laboratory environments.
The immunoassays category is projected to expand rapidly in the laboratory products and outsourcing services market in the coming years, because immunoassays utilize antibodies to identify specific antigens, making them essential for diagnosing diseases such as cancer, cardiovascular issues, and infections. Advancements in immunoassay technologies, including chemiluminescent immunoassays (CLIA), enzyme-linked immunosorbent assays (ELISA), and multiplex assays, have enhanced efficiency and decreased turnaround times in clinical environments. The growing incidence of chronic and infectious illnesses, improvements in laboratory automation, and partnerships among firms have enhanced the functionalities and scope of immunoassay technologies.
End-Use Insights
The medical device segment dominated the laboratory products and outsourcing services market with the highest share in 2024 due to rising demand and technological progress. These firms encounter strict regulatory demands that necessitate high-quality standards and safety measures. Outsourcing services offer specialized skills and advanced technologies without large internal expenditures, allowing businesses to effectively achieve these standards. This strategic method optimizes operations and facilitates quicker market entry for new devices, aiding in the leadership of the market in the medical device companies.
- In October 2024, NAMSA and TERUMO established a strategic alliance to accelerate regulatory approval for a range of medical devices by TERUMO, emphasizing the dependency of industry on outsourcing for market access.
The pharmaceutical and biotech companies' segment is projected to grow with the fastest CAGR in the market during the forecast period, as they concentrate on research and development to create and introduce new treatments to the market. The timeline for developing these products is sped up by outsourcing services, enabling companies to utilize advanced technologies and skilled experts without the significant expenses linked to sustaining internal resources. This strategic method enables them to focus on essential strengths while speeding up the process of developing new medications.
Regional Insights
U.S. Laboratory Products and Outsourcing Services Market Size and Growth 2025 to 2034
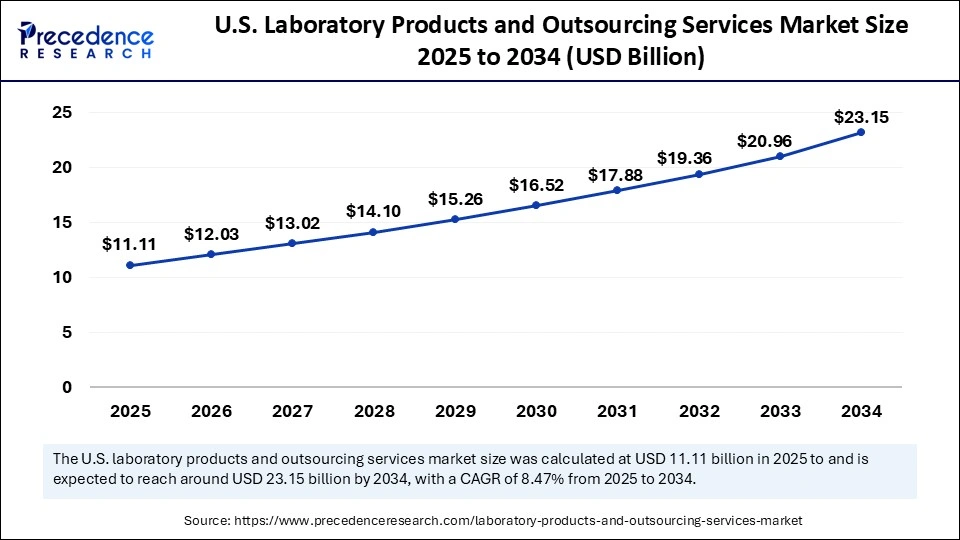
The U.S. Laboratory Products and Outsourcing Services market size is exhibited at USD 11.11 billion in 2025 and is projected to be worth around USD 23.15 billion by 2034, growing at a CAGR of 8.47% from 2025 to 2034.
How North America Stays Ahead in the Market
North America dominated the laboratory products and outsourcing services market in 2024, because of its strong healthcare infrastructure, substantial investment inResearch and Development, and prominent presence of top pharmaceutical and biotechnology firms. The sophisticated technological environment, strict regulatory standards, and superior quality benchmarks of the area enhance its market leadership. The U.S. represents a significant share of this market, bolstered by its established pharmaceutical sector and favorable regulatory landscape. The sophisticated healthcare system, substantial number of clinical trials, and stringent regulatory standards of the region guarantee high-quality and dependable laboratory operations, motivating companies to outsource services to fulfill compliance obligations.
- In May 2024, CPHI North America 2024 took place, drawing industry leaders, innovators, and developers to present advancements in pharmaceutical and laboratory services, emphasizing the dedication to innovation and collaboration of the region.
The U.S. Laboratory Products and Outsourcing Services Market Trends
The laboratory products and outsourcing services market is led by the U.S., due to its sophisticated healthcare system, substantial clinical trial activity, robust biotech and pharmaceutical sectors, considerable government backing, and favorable regulatory conditions. The U.S. boasts advanced healthcare facilities and research institutions, fostering innovation and increasing the demand for laboratory products and services. Key companies such as Pfizer, Merck, and Moderna significantly invest in research and development, frequently outsourcing their laboratory operations. The FDA implements strict quality and safety regulations, requiring companies to partner with specialized service providers.
Cost-Effectiveness and Talent Propel Growth in Asia Pacific
The Asia Pacific region is anticipated to grow at the fastest rate in the market during the forecast period. The region has experienced considerable progress in the pharmaceutical and biotechnology industries, driven by heightened R&D funding, an increasing array of clinical trials, and a surge in the need for specialized laboratory services.
- In March 2025, AstraZeneca intends to invest USD 2.5 billion in a research and development center in Beijing over the next five years, collaborating with local biotech companies to enhance early-stage research and clinical development, despite facing regulatory hurdles.
The reduced operational and labor expenses in nations such as China and India render it appealing for outsourcing laboratory services, enabling firms to minimize costs while maintaining quality. Government programs and a talented workforce in the science and technology sectors also enhance the strengths of the region in biotechnology and pharmaceutical research.
- In 2023, 55% of global trials included a trial site in Asia Pacific, and since 2021, the number of trials initiated in the region has exceeded that of other regions, requiring substantial laboratory services to assist research efforts.
Japan: Enhancing Precision Medicine through Innovative Advancements
Japan is becoming a crucial participant in the laboratory products and outsourcing services market due to its sophisticated healthcare infrastructure, robust governmental backing for research and development, and an emphasis on precision medicine. Due to a significant occurrence of chronic illnesses and an elderly demographic, there has been a rise in the need for creative treatments and diagnostic technologies. Regulatory framework and governmental efforts of Japan, such as the Society 5.0 initiative, enhance its market standing.
Government Funding Drives Pharmaceutical Growth in China
China is witnessing swift expansion in the market due to its expanding pharmaceutical and biotechnology industries, governmental funding in healthcare and research, along with projects such as the Made in China 2025 initiative. The affordable workforce and regulatory changes of the nation, including the National Medical Products Administration, position it as a favored location for clinical trials and laboratory outsourcing. The rising emphasis on personalized medicine and an expanding middle-class demographic pursuing improved healthcare additionally bolsters its market leadership.
Regulatory Excellence Positions Europe as the Market Champion
Europe plays an important role in the laboratory products and outsourcing services market because of its robust healthcare infrastructure, adherence to regulations, and emphasis on research. The cooperative atmosphere among stakeholders, such as government agencies, educational organizations, and private enterprises of the region, promotes progress in laboratory technologies and services. The market is anticipated to expand because of rising healthcare investments and a greater need for diagnostic services. The European Medicines Agency upholds rigorous quality standards, fostering a need for sophisticated laboratory services. Emphasis of Europe on tailored medicine and sustainable chemistry aligns with international trends, positioning it as a key participant in the market.
- In December 2023, the European Commission appointed five European labs as EU Reference Laboratories for high-risk in vitro diagnostic medical devices, improving the quality and reliability of diagnostic testing.
Why the UK Laboratory Products and Outsourcing Services Market is Experiencing Rapid Growth
The UK laboratory products and outsourcing services market is expanding due to increasing demand for advanced research solutions, rising R&D investments in pharmaceuticals and biotechnology, and the need for cost-effective, specialized services. Outsourcing clinical trials, testing, and analytical services allows organizations to focus on core activities while leveraging external expertise. Additionally, government support, technological advancements, and a growing emphasis on efficiency and accuracy are driving market growth.
Top Vendors and their Offerings
- Labcorp: Provides comprehensive clinical laboratory testing, drug development services, diagnostics, and laboratory outsourcing solutions to support pharmaceutical, biotech, and healthcare research.
- SGS SA: Offers laboratory testing, inspection, certification, and compliance services across pharmaceuticals, life sciences, and industrial sectors, ensuring quality, safety, and regulatory adherence.
PerkinElmer: Supplies laboratory instruments, reagents, and services for diagnostics, environmental testing, and life sciences research, focusing on innovation and efficiency in analytical workflows. - Agile Technologies: Delivers specialized laboratory outsourcing services, including analytical testing, R&D support, and technical solutions to streamline research and development processes.
- WuXi AppTec, Inc.: Provides end-to-end pharmaceutical, biotech, and medical device R&D outsourcing, including laboratory testing, preclinical and clinical development, and manufacturing services.
Laboratory Products and Outsourcing Services Market Companies

- Thermo Fisher Scientific
- Charles River Laboratories
- Eurofins Scientific
- Labcorp
- Quest Diagnostics
- Parexel International Corporation
- Agilent Technologies
- PerkinElmer
- Bio-Rad Laboratories
- SGS SA
- Intertek Group plc
- WuXi AppTec, Inc
Leaders' Announcements
- In December 2024, Sonic Healthcare, an Australian pathology firm, acquired Laboratory Group Dr. Kramer and Colleagues for 423 million euros. The acquisition intends to enhance the footprint of Sonic in the European market and is projected to raise earnings per share.
- In November 2024, Cinven, an Italian private equity firm, received conditional approval to sell a 15% share in Synlab to Labcorp, representing a notable step in the consolidation of the laboratory services industry.
Recent Developments
- In October 2023, the National Institutes of Health (NIH) allocated USD 500 million to upgrade laboratory facilities throughout the U.S., focusing on fostering advanced research in genomics, proteomics, and drug development.
- In June 2024, Labcorp teamed up with Thermo Fisher Scientific to enhance its abilities in high-throughput genomic testing. This partnership seeks to improve clinical trial assistance services and streamline processes in precision medicine.
- In February 2025, PerkinElmer introduced an AI-powered laboratory automation system to enhance workflows in sample preparation and data analysis. This technology greatly shortens turnaround times and improves reproducibility in studies.
Segments Covered in the Report
By Type
- Products
- Equipment
- General
- Analytical
- Clinical
- Support
- Specialty
- Disposables
- Others
- Equipment
- Services
- Pharmaceutical
- Bioanalytical Testing
- ADME
- PK
- PD
- Bioavailability
- Bioequivalence
- Others
- Pharmaceutical
- Method Development and Validation
- Extractable and Leachable
- Impurity Method
- Technical Consulting
- Others
- Stability Testing
- Drug Substance
- Stability Indicating Method Validation
- Accelerated Stability Testing
- Photostability Testing
- Others
- Others
- Medical Device
- Biocompatibility Tests
- Chemistry Test
- Microbiology and Sterility Testing
- Others
- Medical Device
By Technology
- Immunoassays
- Molecular Diagnostics
- Microbiology
- Clinical Chemistry
- Flow Cytometry
- Mass Spectroscopy
- Chromatography
- Others
By End-Use
- Pharmaceutical and Biotech Companies
- Medical Device Companies
- CRO and CDMO
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting