What is Nanotechnology in Medical Devices Market?
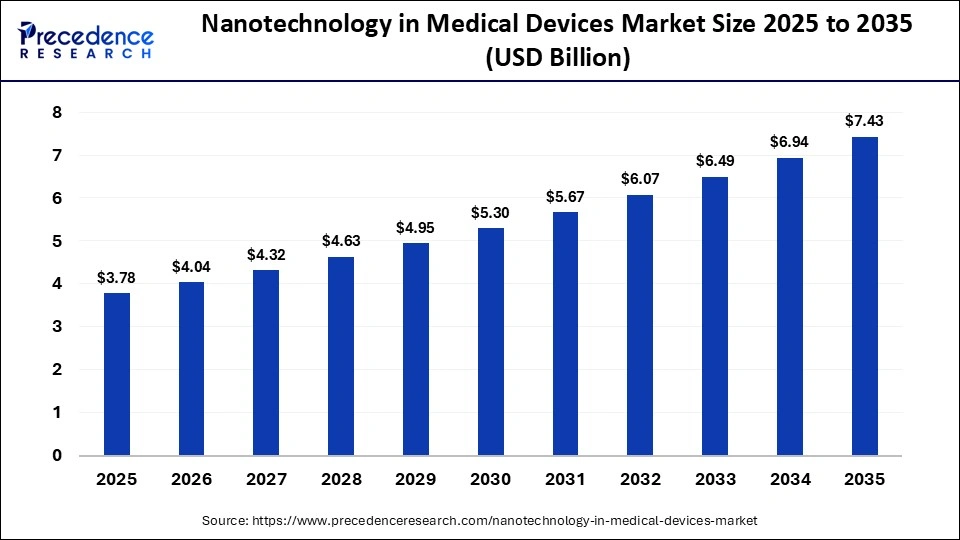
The global nanotechnology in medical devices market size was calculated at USD 3.78 billion in 2025 and is predicted to increase from USD 4.04 billion in 2026 to approximately USD 7.43 billion by 2035, expanding at a CAGR of 7.00% from 2026 to 2035. The market is advancing rapidly due to rising demand for minimally invasive procedures, improved diagnostics, and targeted therapies. Nano-enabled coatings, sensors, and implants enhance device performance, biocompatibility, and patient outcomes across healthcare applications.
Market Highlights
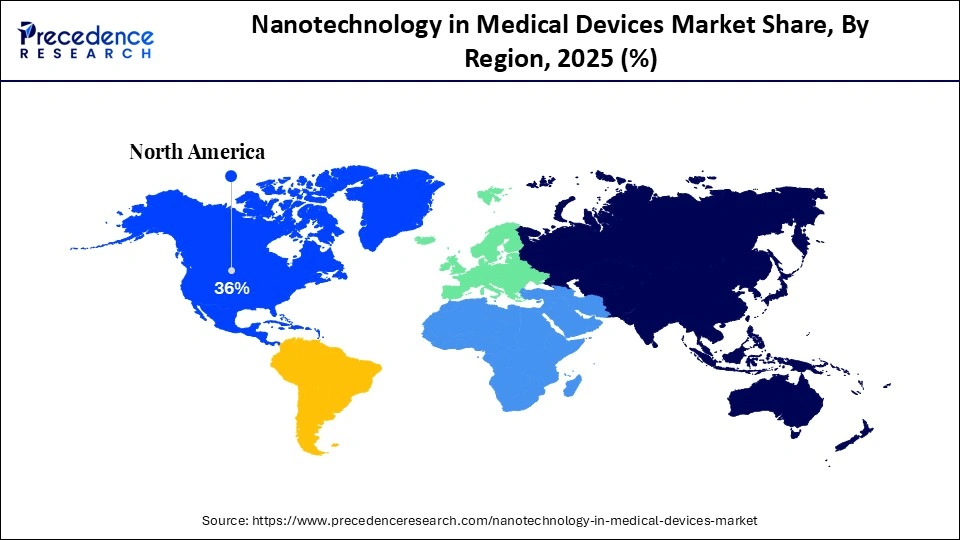
- North America led the nanotechnology in medical devices market with the largest market share of 36% in 2025.
- Asia-Pacific is expected to expand at the highest CAGR in the market during the forecast period.
- By type, the implantable devices segment held a dominant revenue share of the market in 2025.
- By type, the wound care segment is expected to grow at the highest CAGR between 2026 and 2035.
- By application, the orthopedics segment held the largest revenue share of the market in 2025.
- By application, the dentistry segment is expected to expand at a notable CAGR from 2026 to 2035.
- By end use, the hospitals segment held a the major market share of 51% in 2025.
- By end use, the clinics segment is projected to grow at a solid CAGR in the coming years.
How Is Nanotechnology Transforming the Future of Medical Devices?
Nanotechnology is transforming the future of medical devices through the integration of nanoscale materials and engineering to enhance device precision, functionality, and safety. Nano-enabled coatings, sensors, and implants improve diagnostics, drug delivery, and treatment outcomes. Growing adoption of minimally invasive procedures and advanced healthcare technologies is driving strong growth across the nanotechnology medical devices industry.
How is AI Transforming the Nanotechnology in Medical Devices Market?
Artificial intelligence (AI) is transforming nanotechnology-based medical devices by improving design, optimization, and application outcomes through data-driven, predictive algorithms. AI enhances nanosensor accuracy in clinical diagnostics by interpreting complex biological signals and reducing false positives, enabling earlier disease detection. It also guides nanoparticle design and fabrication processes, optimizing material properties for targeted drug delivery and personalized therapeutics.
In neurodegenerative disease management, AI improves nanodiagnostic sensitivity and patient stratification, enabling real-time monitoring and personalized care pathways. These capabilities accelerate clinical translation and operational performance of nano-enabled devices while addressing complex data interpretation challenges in healthcare settings.
- For instance, In December 2025, at RSNA 2025, HOPPR, a company dedicated to revolutionizing the development of AI for medical imaging, announced the introduction of two new initiatives for the HOPPRTM Al Foundry: the Catalyst Program and Forward Deployed Services (FDS). When combined, they increase access to foundation models, high-quality imaging datasets, and practical expert support, providing organizations with flexible ways to accelerate research and development in medical imaging.
Primary Trends Influencing the Nanotechnology in Medical Devices Market
- Nano-enabled Drug Delivery Systems: Nanotechnology is advancing targeted drug delivery devices that precisely transport therapeutics to specific cells or tissues. Nanoscale carriers improve bioavailability, reduce side effects, and allow controlled release. These systems enhance treatment effectiveness, especially in oncology and chronic diseases, by minimizing systemic exposure and improving patient adherence through smarter, responsive nanostructures.
- Nanosensors for Real-Time Monitoring: Nanoscale sensors are being integrated into wearable and implantable devices to continuously monitor physiological parameters like glucose, biomarkers, and vital signs. Their high sensitivity enables earlier detection of abnormalities and dynamic feedback. This trend supports personalized health tracking, faster clinical decision-making, and remote patient management using miniaturized, high-precision nanotechnology components.
- Nano-coatings for Antimicrobial Protection: Medical devices are increasingly using nanocoatings with antimicrobial properties to reduce infection risk. Nanostructured surfaces can disrupt bacterial adhesion, prevent biofilm formation on implants, and enhance sterilization. These coatings enhance device lifespan and patient safety, particularly in surgical instruments, catheters, and prosthetics, contributing to better clinical outcomes and reduced healthcare-associated infections.
- Nanostructured Biomaterials for Tissue Engineering: Nanotechnology is enabling the development of biomaterials with architected nanostructures that mimic natural tissue environments. These materials support cell growth, differentiation, and integration for regenerative therapies. Used in bone, cartilage, and soft tissue repair, nanostructured materials improve biocompatibility and mechanical strength, helping create next-generation implants that integrate seamlessly with the body.
- Nanorobotics and Micro-actuators: Emerging nanorobotic devices and micro-actuators are being engineered to perform intricate tasks within the human body, such as navigating narrow pathways or administering localized therapies. These tiny machines, driven by nanoscale components, promise precision interventions with minimal invasiveness, opening possibilities for autonomous diagnostics and targeted microsurgery guided by nanotechnology breakthroughs.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 3.78 Billion |
| Market Size in 2026 | USD 4.04 Billion |
| Market Size by 2035 | USD 7.43 Billion |
| Market Growth Rate from 2026 to 2035 | CAGR of 7.00% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | By Type,Application,End Use, and region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Segmental Outlook
Type Insights
Why the Implantable Devices Segment Dominated the Market?
The implantable devices segment held a dominant position in the nanotechnology in medical devices market in 2025, as nanotechnology enhances implantable medical devices by improving biocompatibility, reducing inflammation and infection through nano-engineered surfaces that mimic natural tissues, promoting cell growth and integration with surrounding bone or organs.
These nanoscale modifications also improve mechanical strength, electrical conductivity, and long-term stability, making implants more effective, durable, and safer for chronic conditions and continuous monitoring inside the body. The increasing launch and commercialization of implantable nanotechnology in medical devices fosters the segment's growth.
- For instance,In 2025, Nanovis, an Indiana-based medical technology company, commercialized its nanoVIS Ti surface technology, an FDA-designated nanotechnology surface, applied to orthopedic, spine, and dental implants. This nano-engineered surface was designed to enhance bone growth and osseointegration while reducing infection risk.
The wound care segment is expected to grow at the fastest CAGR in the market between 2026 and 2035 because nanomaterials can directly interact with biological systems at the cellular and molecular level, improving healing and infection control where conventional dressings fail. Their high surface area-to-volume ratio enhances antibacterial activity, sustained drug release, moisture balance, and tissue regeneration, supporting faster closure and reduced complications in acute and chronic wounds. These nano-engineered dressings can also modulate inflammation, promote angiogenesis, and mimic natural tissue environments to accelerate healing more effectively than traditional materials.
Application Insights
Which Application Segment Dominated the Nanotechnology in Medical Devices Market?
The orthopedics segment registered its dominance over the global market in 2025, as nanomaterials significantly enhance implant performance where traditional materials struggle. Nanoscale surface features and coatings improve bone cell adhesion, osseointegration, and structural mimicry of natural bone, reducing loosening and infection risk. These advances also boost mechanical strength, wear resistance, and tissue regeneration, making orthopedic devices more durable, safer, and clinically effective for long-term use.
The dentistry segment is expected to grow significantly in the market in the coming years due to the ability of nanomaterials to dramatically improve dental care effectiveness and patient outcomes. Nano-enhanced composites and ceramics strengthen restorations, enhance aesthetics, and resist wear better than traditional materials. Antimicrobial nanoparticles reduce bacterial growth and prevent decay, while nanostructured surfaces on implants improve osseointegration and healing, making dental treatments more durable, comfortable, and precise. These innovations support better preventive care, diagnostics, and restorative success in everyday dental practice.
End Use Insights
What Made Hospitals the Dominant Segment in the Market?
The hospitals segment held the largest revenue share in the nanotechnology in medical devices market in 2025 because hospital settings demand advanced diagnostics, infection control, and precision therapies that nanoscale technologies uniquely provide. In hospitals, nanotechnology-based devices enhance pathogen detection sensitivity, improve antimicrobial surfaces and coatings to help combat healthcare-associated infections, and support targeted drug delivery and real-time monitoring capabilities essential for effective acute care and complex treatments. This integration of nano-innovations into hospital practices improves patient safety, accelerates diagnosis and treatment, and addresses critical challenges like antimicrobial resistance in healthcare environments.
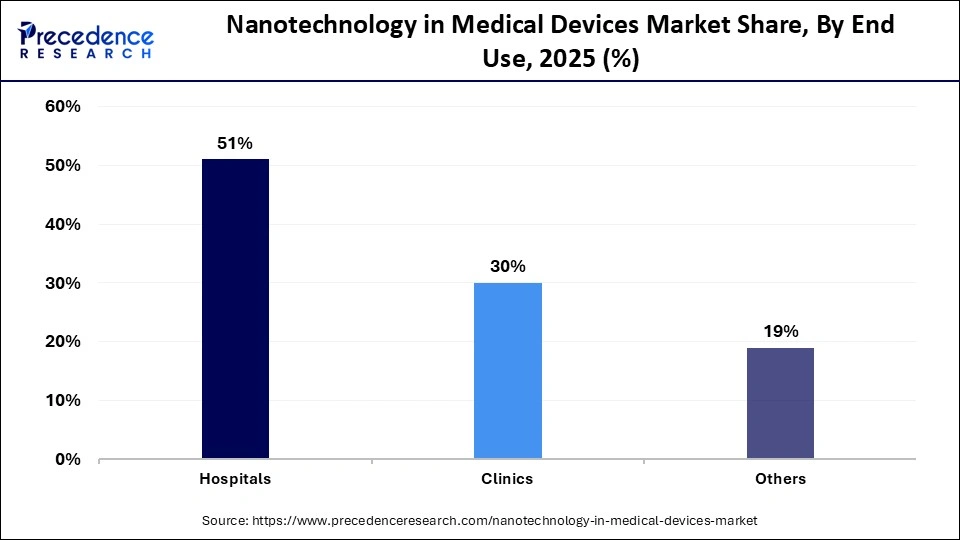
The clinics segment is expected to gain the highest share of the market over the studied period. Nanotechnology accelerates clinics' growth by enabling portable, highly sensitive point-of-care (POC) diagnostic tools that deliver rapid, accurate results outside central labs. Nano-enabled sensors and lab-on-chip systems detect diseases at very low biomarker levels, aiding early diagnosis and treatment in outpatient settings. These technologies make advanced testing more accessible and practical in clinics, improving patient care and reducing delays.
Regional Insights
How Big is the North America Nanotechnology in Medical Devices Market Size?
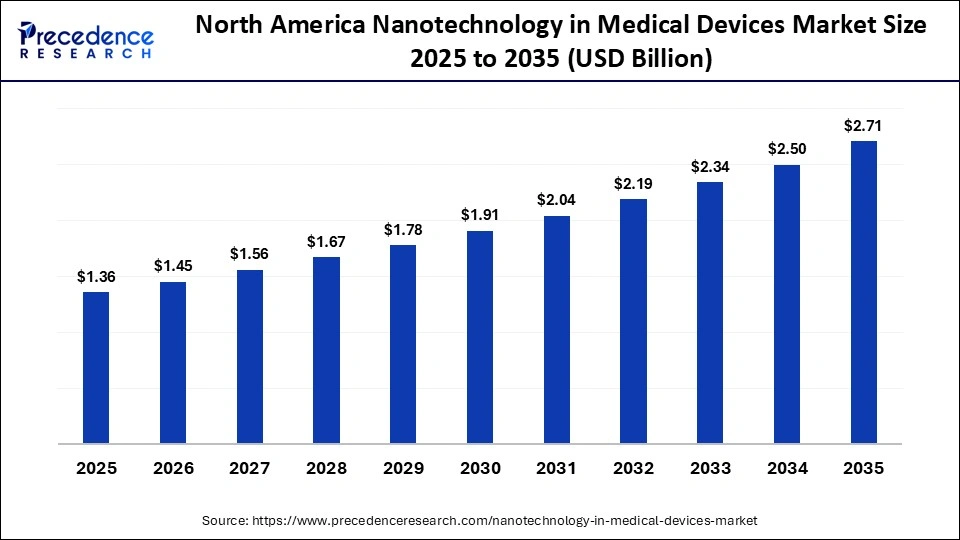
The North America nanotechnology in medical devices market size is estimated at USD 1.36 billion in 2025 and is projected to reach approximately USD 2.71 billion by 2035, with a 7.14% CAGR from 2026 to 2035.
Why North America Dominated the Nanotechnology in Medical Devices Market?
North America held a major revenue share of the market in 2025, because the U.S. and Canada have well-established research and clinical ecosystems that actively develop and adopt advanced nanotechnology solutions. Strong federal initiatives like the National Nanotechnology Initiative (NNI) support coordinated nanoscale research and commercialization efforts. The region's advanced healthcare infrastructure, extensive R&D collaboration between academia and medical institutions, and high clinical trial activity accelerate integration of nano-enabled diagnostics and therapies into practice. This combination of robust scientific support and clinical adoption sustains North America's dominant position.
What is the Size of the U.S. Nanotechnology in Medical Devices Market?
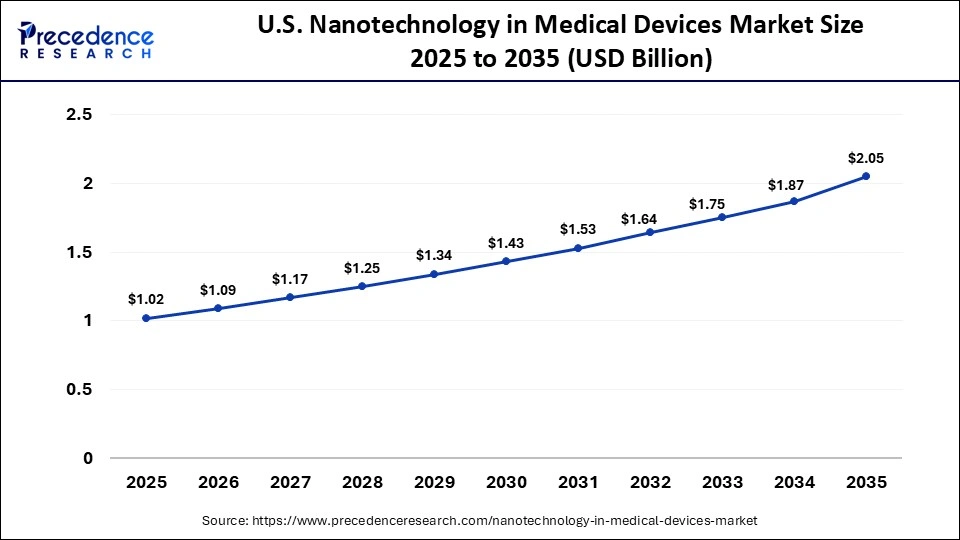
The U.S. nanotechnology in medical devices market size is calculated at USD 1.02 billion in 2025 and is expected to reach nearly USD 2.05 billion in 2035, accelerating at a strong CAGR of 7.23% between 2026 to 2035.
U.S. Market Trends
The U.S. has emerged as the dominant country in North America mainly due to robust federal coordination and funding for nanoscale research and commercialization, especially through the National Nanotechnology Initiative (NNI), which aligns multiple agencies to support innovation and product development. The U.S. benefits from world-class universities and research centers, abundant research infrastructure, and extensive collaborations between government, academia, and industry that accelerate translational nanomedicine research into clinical applications and strengthen leadership in advanced medical device technologies.
Which Factors Drive the Nanotechnology in Medical Devices Market in Asia-Pacific?
Asia-Pacific is expected to host the fastest-growing market in the coming years because of strong government support and funding for nanotech research, increasing healthcare expenditure, and expanding clinical adoption of advanced nanoscale diagnostics and therapeutics. Large patient populations with rising chronic disease burdens and collaborations between industry and academic institutions further accelerate innovation and market uptake. Evolving regulatory landscapes, growing emphasis on indigenous manufacturing, and the rising adoption of advanced technologies propel market growth.
China Market Trends
China holds a major share in Asia-Pacific due to aggressive government support for nanotech R&D and commercialization, a high volume of domestic patents, and robust industry-academia collaboration driving innovative device development and regulatory acceleration. Key trends include growing focus on targeted drug-delivery systems, advanced nanosensors for early diagnostics, nanorobotics research, and rising clinical adoption of nano-enabled medical solutions aimed at precision medicine and improved patient outcomes.
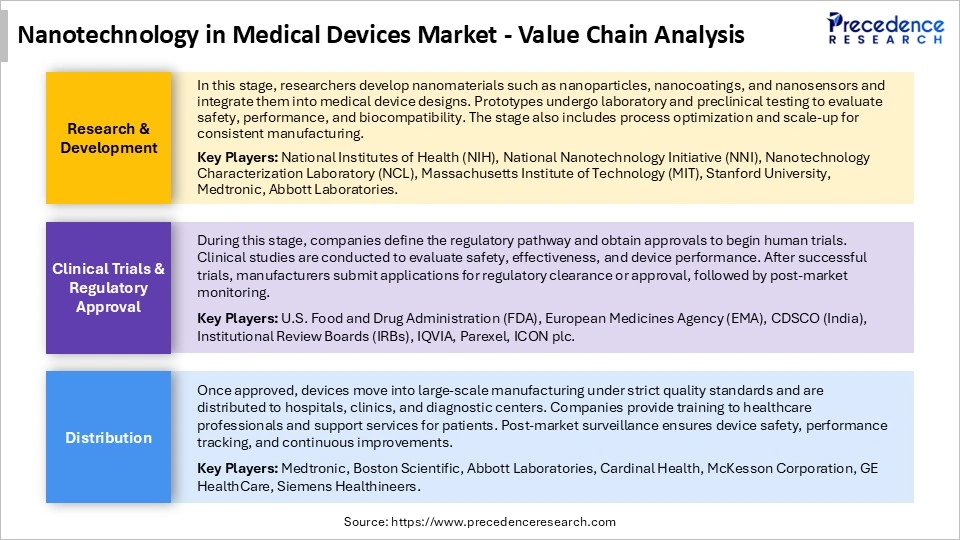
Nanotechnology in Medical Devices Market Value Chain Analysis
Who are the major players in the global nanotechnology in medical devices market?
The major players in the nanotechnology in medical devices market include Thermo Fisher Scientific Inc., Medtronic plc, 3M Company, Stryker Corporation, Smith & Nephew PLC, PerkinElmer Inc., General Electric Company (GE), Dentsply Sirona, Boston Scientific Corporation, Ferro Corporation, Eppendorf AG, TÜV Rheinland AG, BIOTRONIK SE & Co. KG, LivaNova PLC, Cochlear Ltd., Sonova Group, MED-EL Medical Electronics, Zimmer Biomet Holdings Inc, and Abbott Laboratories
Recent Developments in the Nanotechnology in Medical Devices Market
- In October 2025, Flinders University researchers reported a novel liquid metal-based scaffold with embedded silver-gallium nanoparticles that combats infection and enhances bone regeneration. This dual-function nanoscale biomaterial, tested in preclinical models, offers sustained antimicrobial activity and improved osseointegration compared to conventional implant materials.[Source: https://news.flinders.edu.au]
- In March 2025, Medtronic plc announced the acquisition of Nanovis' proprietary nano surface technology designed to improve bone fixation and biological integration in spinal, dental, and orthopedic implants. The integration of nanoscale surface helps enhance osseointegration, reduce bacterial attachment, and accelerate recovery, with Medtronic aiming to incorporate the technology across next-gen PEEK interbody fusion and trauma implants.[Source: https://thehealthcaretechnologyreport.com)
- In October 2024, UPM Biomedicals announced the launch of FibGel, a natural injectable hydrogel for permanent implantable medical devices. FibGel is a nanofibrillar cellulose hydrogel that offers a safe, sustainable, and biocompatible alternative for medical device developers in various applications, including tissue repair, orthopedics, and regenerative medicine. (Source: https://www.azom.com)
Segments Covered in the Report
By Type
- Implantable Devices
- Orthopedic Implants
- Hearing Aids
- Cardiac Rhythm Management Devices
- Others
- Dental Filling Materials
- Wound Care
- Others
By Application
- Dentistry
- Orthopedics
- Hearing Loss
- Wound Care
- Others
By End Use
- Hospitals
- Clinics
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting