What is the Neurointerventional Devices Market Size?
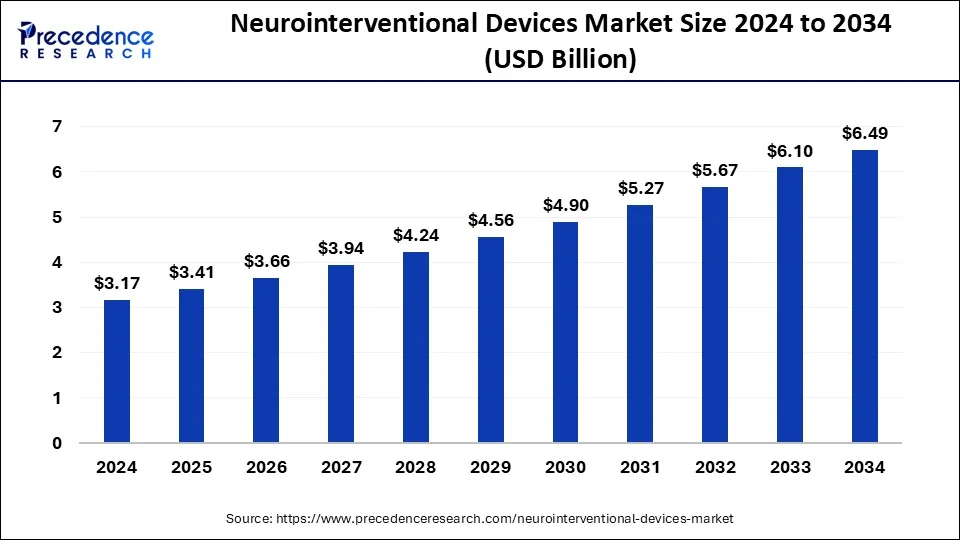
The global neurointerventional devices market size was estimated at USD 3.41 billion in 2025 and is predicted to increase from USD 3.66 billion in 2026 to approximately USD 6.91 billion by 2035, expanding at a CAGR of 7.32% from 2026 to 2035. Rising demand for minimally invasive procedures, coupled with a growing aging population more susceptible to neurological diseases, are driving the market's growth further.
Neurointerventional Devices Market Key Takeaways
- The global neurointerventional devices market was valued at USD 3.41 billion in 2025.
- It is projected to reach USD 6.91billion by 2035.
- The neurointerventional devices market is expected to grow at a CAGR of 7.32% from 2026 to 2035.
- North America dominated the global market with the largest market share of 36% in 2025.
- Asia Pacific is projected to experience significant growth in the upcoming years.
- By product, the catheter segment contributed the highest market share in 2025.
- By indication, the stroke segment has held a major market share of 61% in 2025.
- By indication, the brain aneurysm segment is emerging as one of the fastest-growing segments in the market.
- By application, the hospital segment captured the biggest market share of 48% in 2025.
- By application, the ambulatory surgical center (ASC) segment is rapidly emerging as one of the fastest-growing segments in the market.
What is the Neurointerventional Device?
The neurointerventional device market is experiencing significant growth due to technological advancements and the increasing prevalence of neurological disorders. These devices include stents, embolic coils, and thrombectomy devices, which are crucial in treating medical conditions such as strokes, aneurysms, and arteriovenous malformations.
The market is driven by rising demand for minimally invasive procedures, coupled with a growing aging population more susceptible to neurological diseases. Additionally, expanding healthcare infrastructure and improving reimbursement policies further propel market expansion. Key players in the industry are focusing on innovation, strategic partnerships, and geographical expansion to gain a competitive edge. With continuous research and development efforts, the neuro interventional devices market is poised for sustained growth in the foreseeable future.
How is AI contributing to the Neurointerventional Devices Industry?
AI is used to strengthen neurointerventional devices, improving the image quality, precision of navigation, and clinical decision-making. It is capable of tracking catheterization in real-time, searching and identifying strokes automatically, modeling complications in advance, and assisting robots. Complex interventions involving the neurovascular system are made faster, safer, and more individualized.
Neurointerventional Devices Market Growth Factors
- Advancements in technology are driving innovation in neurointerventional devices.
- Increasing prevalence of neurological disorders, such as strokes and aneurysms.
- Growing demand for minimally invasive procedures for neurological treatments.
- Rapid Expansion of healthcare infrastructure, particularly in emerging markets.
- Improving reimbursement policies for neurointerventional procedures.
- The rising aging population globally is increasing the pool of patients requiring neurological interventions.
- Strategic partnerships and collaborations among key industry players fostering market growth.
- Continuous research and development efforts aimed at enhancing the efficacy and safety of neurointerventional devices.
- Geographical expansion strategies to tap into new markets and segments.
- Heightened awareness about the benefits of neurointerventional procedures among both patients and healthcare professionals.
Market Outlook
- Industry Growth Overview: The Industry Development: Increasing cases of stroke and aneurysms drive the adoption of minimally invasive neurointerventional technologies faster.
- Sustainability Trends: The manufacturers minimize procedural waste, enhance the efficiency of packing, and use environmentally friendly materials.
- Major Investors: Acquisitions and innovation are driven by Medtronic, Stryker, Johnson & Johnson, Penumbra, and Terumo.
Market Scope
| Report Coverage | Details |
| Global Market Size in 2025 | USD 3.41Billion |
| Global Market Size in 2026 | USD 3.66 Billion |
| Global Market Size by 2035 | USD 6.91Billion |
| Market Growth Rate from 2026 to 2035 | CAGR of 7.32% |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | By Product, By Indication, and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Rising neurological disorders
One significant driver for the neurointerventional devices market is the increasing prevalence of neurological disorders, such as strokes and aneurysms. As populations age and lifestyles change, the incidence of neurological conditions continues to rise globally. Strokes are a leading cause of death and disability, prompting a greater emphasis on early intervention and treatment. The growing awareness about the importance of timely and effective treatment for neurological conditions has led to an upsurge in demand for neurointerventional devices. These devices offer minimally invasive solutions that can help mitigate the effects of strokes, aneurysms, and other neurological emergencies.
- According to the World Health Organization (WHO), neurological disorders affect millions of people worldwide, contributing to significant morbidity and mortality rates. For instance, according to the new data released by Lancet Neurology, more than 3 billion people around the globe are dealing with neurological conditions.
Advancements in imaging technology and surgical techniques have improved the precision and efficacy of neurointerventional procedures, making them more accessible and safer for patients. Hence, the increasing prevalence of neurological disorders underscores the critical need for neurointerventional devices, driving market growth as healthcare providers seek innovative solutions to address the growing burden of neurological diseases.
Restraint
Cost barrier
A significant restraint in the neurointerventional devices market is the high cost associated with these advanced medical technologies. Neurointerventional procedures often require specialized equipment, such as stents, embolic coils, and thrombectomy devices, which can be expensive to manufacture and procure. Additionally, the need for trained healthcare professionals to perform these procedures further adds to the overall cost of treatment.
The high cost of neurointerventional devices poses a challenge, particularly in regions with limited healthcare resources or where reimbursement policies are inadequate. Patients may face financial barriers to accessing these life-saving interventions which adversely affects the outcomes of medical procedures. Moreover, healthcare providers and institutions may struggle to invest in the latest neurointerventional technologies due to budget reasons. It is potentially limiting the availability of advanced treatments for patients in need.
Opportunity
Neurostimulation technologies
One future opportunity in the neurointerventional devices market lies in the development of next-generation neurostimulation technologies for the treatment of neurological disorders. Neurostimulation involves the use of implanted devices to modulate neural activity, offering potential therapeutic benefits for conditions such as Parkinson's disease, epilepsy, chronic pain, and psychiatric disorders. For instance, the non-invasive vagus nerve stimulation method is widely used for stress relief. Recent scientific studies have shown that electrical stimulation of the vagus nerve can achieve the same state of calm as prolonged meditation or taking anti-anxiety medications.
Advancements in neurostimulation technology, such as improved electrode designs, novel stimulation waveforms, and enhanced targeting algorithms, hold promise for more precise and effective treatment results with fewer side effects. Additionally, the integration of artificial intelligence (AI) and machine learning algorithms could optimize stimulation parameters and personalize treatment regimens based on individual patient responses and disease characteristics. As the understanding of neural circuitry and brain function continues to advance, there is immense potential for neurostimulation to revolutionize the treatment landscape for neurological disorders, providing patients with safer, more effective, and personalized therapeutic options.
Furthermore, expanding applications of neurostimulation beyond traditional indications could open new markets and revenue streams for neurointerventional device manufacturers. For instance, ongoing research is exploring the use of neurostimulation for cognitive enhancement, mood regulation, and neurological rehabilitation, offering potential opportunities for innovation and growth. Therefore, investing in research and development efforts to further refine and expand neurostimulation technologies represents a promising future opportunity for the neurointerventional devices market.
Segment Insights
Product Insights
The catheter segment dominated the neurointerventional devices market and is anticipated to hold its position in the forecasted years. The reason for the growth is the various catheters available on the market that treat different parts of the body. In the field of neurointerventional devices, catheters play a crucial role in treating conditions affecting the brain and nervous system. These specialized catheters are designed to navigate through the blood vessels of the body, including those in the brain, with precision and safety. They can be used to deliver medications, remove blood clots, or even repair damaged blood vessels.
As technology advances, catheters in the neurointerventional devices market continue to improve, offering better outcomes for patients with conditions such as strokes, aneurysms, malformations, etc. These devices play a vital role in modern medicine, enabling doctors to treat complex neurological conditions more effectively. There are various sizes to accommodate different procedures and patient needs. They are typically made from materials like plastic or metal and are equipped with advanced features such as flexible tips and imaging capabilities to help doctors perform procedures with accuracy.
Indication Insights
The stroke segment stands out as a dominant force in the global neurointerventional devices market. A stroke occurs when blood flow to the brain is disrupted, leading to brain cell damage and potentially life-altering consequences. Neurointerventional devices play a critical role in the treatment of strokes, particularly in cases where traditional therapies like medication are insufficient. Devices such as thrombectomy catheters are used to remove blood clots from blocked arteries in the brain, restoring blood flow and preventing further damage.
The demand for neurointerventional devices in the stroke segment continues to grow as the aging population and lifestyle factors contribute to an increasing incidence of strokes globally. Additionally, advancements in technology have led to the development of more sophisticated devices that enable faster and more effective stroke interventions, improving patient outcomes and reducing long-term disability. As a result, the stroke segment remains a key driver of innovation and growth in the neurointerventional devices market, attracting significant investments from healthcare providers, manufacturers, and research institutions aiming to address this critical medical need.
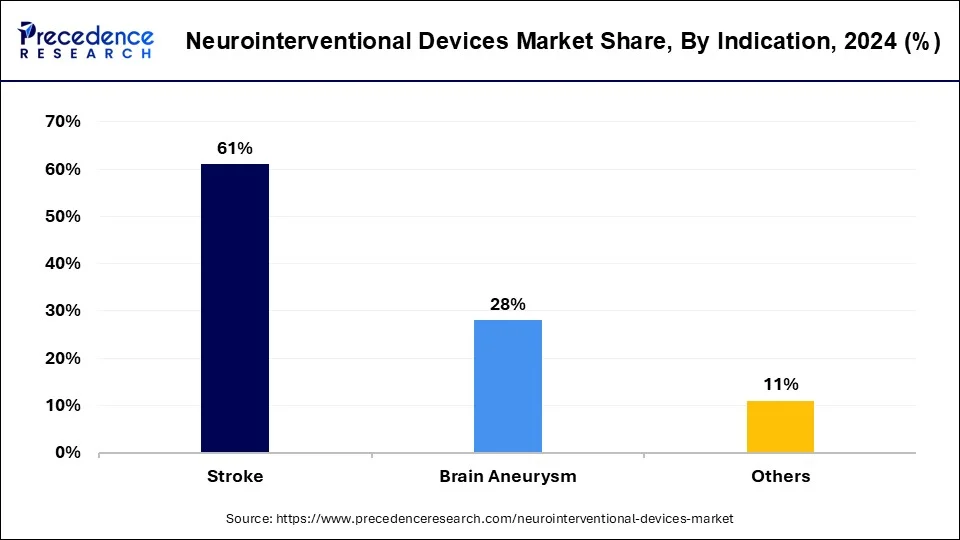
The brain aneurysm segment is emerging as one of the fastest-growing segments in the neurointerventional devices market. Brain aneurysms are abnormal bulges or weak spots in the walls of blood vessels in the brain, which can potentially rupture and cause life-threatening bleeding. Neurointerventional devices are crucial in the treatment of brain aneurysms, offering minimally invasive options to prevent blockages and mitigate the risk of complications. Devices such as stents, coils, and flow diverters are used to reinforce weakened blood vessel walls, divert blood flow away from aneurysms, or block off the aneurysm entirely.
The growing awareness of brain aneurysms, coupled with advancements in diagnostic imaging technologies, has led to increased detection rates and a higher demand for interventional treatments. Moreover, the development of innovative devices with improved safety profiles and efficacy has expanded the treatment options available to patients with brain aneurysms. As a result, the brain aneurysm segment is experiencing rapid growth, with a rising number of procedures performed each year. This trend is expected to continue as healthcare providers and patients recognize the importance of early detection and intervention in preventing potentially hazardous outcomes associated with brain aneurysms.
Application Insights
The hospital segment dominated the neurointerventional devices market in 2025. Hospitals serve as the primary setting for diagnosing and treating patients with neurological conditions requiring neurointerventional procedures. These facilities are equipped with advanced imaging technologies, operating rooms, and specialized medical staff trained in neurointerventional techniques. Hospitals play a pivotal role in the adoption and utilization of neurointerventional devices, as they serve as the main point of care for patients with conditions such as strokes, aneurysms, and malformations. Neurosurgeons, interventional radiologists, and other healthcare professionals rely on these devices to perform minimally invasive procedures that can significantly improve patient outcomes.
The dominance of the hospital segment is further reinforced by factors such as the availability of specialized infrastructure and the ability to provide comprehensive care to patients with complex neurological conditions. Additionally, hospitals often have greater purchasing power and access to resources for investing in cutting-edge neurointerventional technologies, driving market growth and innovation. As healthcare systems continue to prioritize neurointerventional services and invest in expanding their capabilities, the hospital segment is expected to maintain its dominant position in the neurointerventional devices market, driving demand for advanced devices and contributing to improved patient care.
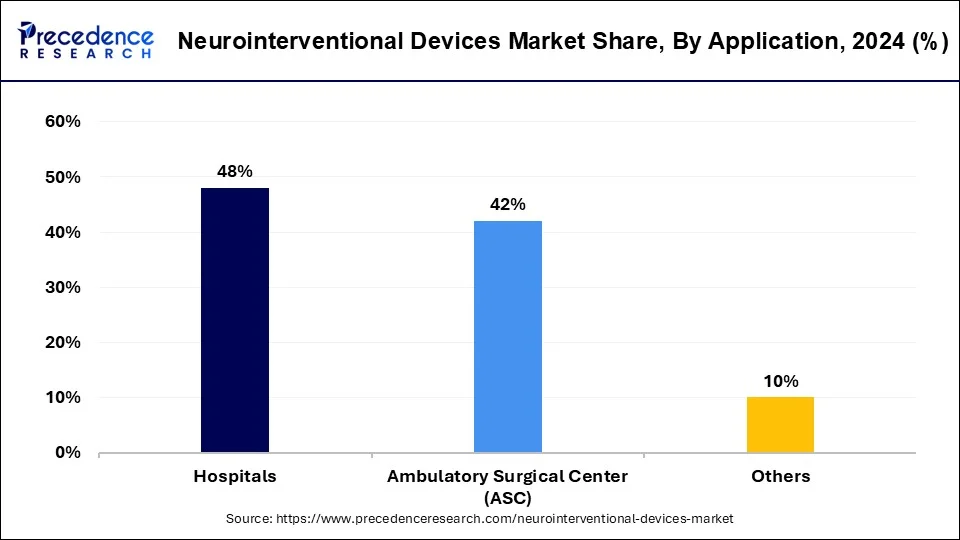
The ambulatory surgical center (ASC) segment is rapidly emerging as one of the fastest-growing segments in the market. ASCs are outpatient facilities that specialize in providing same-day surgical care, including neurointerventional procedures, in a convenient and efficient setting. Advancements in medical technology and techniques have made many neurointerventional procedures feasible in the ambulatory setting, offering several advantages such as shorter recovery times, reduced costs, and decreased risk of hospital-acquired infections. As a result, there is a growing trend toward performing neurointerventional procedures in ASCs, particularly for less complex cases.
ASCs provide an attractive alternative to traditional hospital-based care for both patients and healthcare providers. Patients benefit from the convenience of outpatient procedures, avoiding overnight hospital stays and associated inconveniences. Healthcare providers appreciate the streamlined workflows and cost-effectiveness of ASCs, which often result in quicker times and increased patient health. The rapid growth of the ASC segment in the market is driven by factors such as increasing patient demand for minimally invasive treatments, advancements in outpatient surgical technologies, and reimbursement policies favoring outpatient care. As ASCs continue to expand their capabilities and offerings in neurointerventional procedures, they are poised to play a significant role in shaping the future of neurointerventional care delivery in the neurointerventional devices market.
Regional Insights
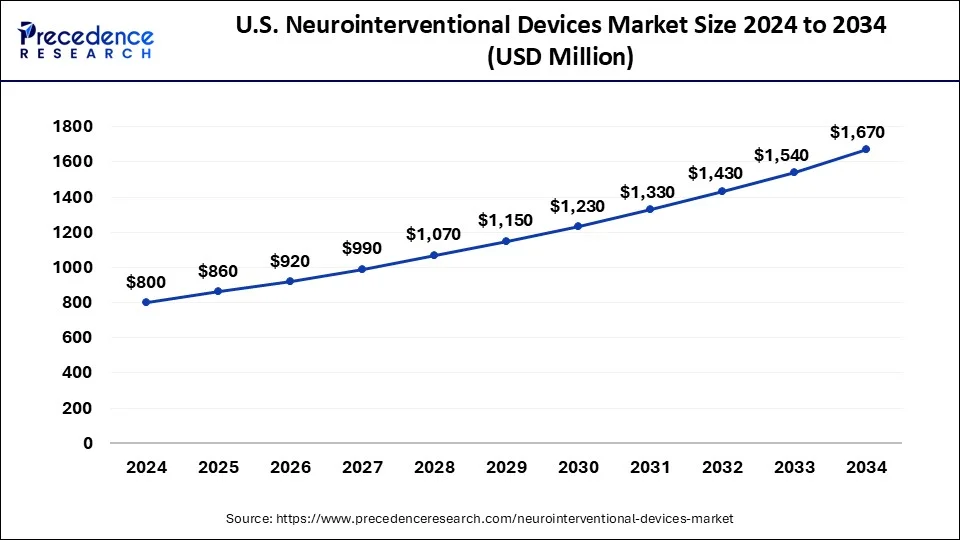
What is the U.S. Neurointerventional Devices Market Size?
The U.S. neurointerventional devices market size surpassed USD 860 million in 2025 and is expected to be worth around USD 1,787 million by 2035 at a CAGR of 7.59% from 2026 to 2035.
North America has dominated the global neurointerventional devices market and is expected to hold its position in the foreseeable future. With advanced healthcare infrastructure and significant investments in medical research and technology, North America leads in the development and adoption of innovative neurointerventional devices.
Hospitals and healthcare facilities across the United States and Canada utilize a wide range of neurointerventional devices, including catheters, stents, coils, and thrombectomy devices, to treat various neurological conditions such as stroke, aneurysms, and arteriovenous malformation.
The presence of leading medical device manufacturers, coupled with favorable reimbursement policies and a growing aging population, further drives the demand for neurointerventional devices in the region. Additionally, ongoing advancements in minimally invasive techniques and image-guided procedures continue to shape the landscape of neurointerventional treatments in North America. Hence, North America remains a key player in the global neurointerventional devices market, setting trends and standards for innovation and patient care.
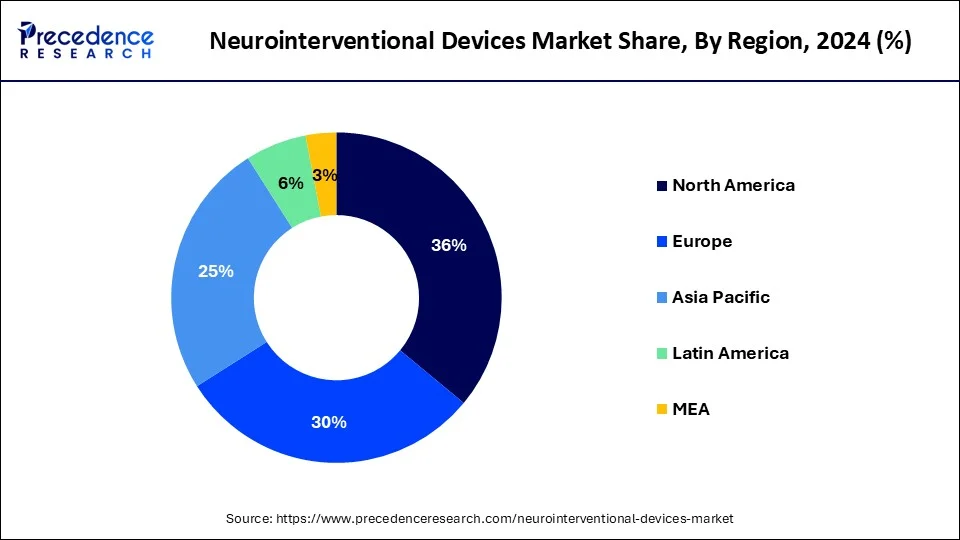
Asia Pacific is projected to experience significant growth in the upcoming years. Asia Pacific represents a dynamic and increasingly important market for neurointerventional devices, with opportunities for both established companies and newcomers to make significant contributions to improving patient outcomes across the region. Rapid urbanization, improving healthcare infrastructure, and increasing healthcare expenditure are contributing to the expansion of neurointerventional procedures across the region.
Countries such as China, Japan, India, South Korea, and Australia are witnessing a rise in the prevalence of neurological disorders, including stroke and aneurysms, leading to a growing demand for advanced neurointerventional devices. Additionally, the aging population and changing lifestyles are further fueling the incidence of neurological conditions, prompting healthcare providers to adopt innovative treatment solutions.
Government initiatives aimed at improving healthcare access and quality, coupled with the expansion of private healthcare facilities, are creating favorable conditions for the adoption of neurointerventional procedures and devices. Furthermore, collaborations between regional healthcare providers and international medical device manufacturers are facilitating technology transfer and the localization of neurointerventional devices to suit the needs of the local population better. These sustain the growth of the neurointerventional devices market in the region.
What Are the Driving Factors of The Neurointerventional Devices Market in Europe?
Europe is expected to grow at a significant rate during the forecast period. Europe demonstrates consistent growth due to harmonized device regulations and government funding of strokes. Flow diverters are adopted in greater numbers. Buying decisions are motivated by clinical evidence. Standard care plans are broadened. Investment in the specialisation of imaging and neurovascular in hospitals takes place in large healthcare systems.
Germany Neurointerventional Devices Market Trends
Germany is evidence-based, with an outcome focus on neurointervention. The focus of demand is on the MDR-compliant devices. Hybrid operating rooms grow. There is an increase in the adoption of high-resolution imaging. The procurement in a hospital becomes centralized. Technology selection and choice are guided by precision, safety, and regulatory compliance in neurovascular treatment facilities.
Value Chain Analysis of the Neurointerventional Devices Market
- R&D: High-value innovation and translation of clinical needs into proprietary neurointerventional device designs and prototypes.
Key players: Medtronic, Stryker, Penumbra, Cerenovus (J&J), Terumo (MicroVention) - Clinical Trials and Regulatory Approvals: Safety and efficacy validation, acquiring approvals from international regulatory agencies for the medicine.
Key players: FDA (US), EMA (EU), NMPA (China), Medtronic, Stryker, Penumbra - Formulation and Final Dosage Preparation: Accurate assembling of biocompatible materials and coatings into sterile functional devices.
Key players: Medtronic, Stryker, Penumbra, Terumo (MicroVention), Balt - Packaging and Serialization: Unique identification in sterile containers that offer traceability and anti-counterfeiting.
Key players: Sharp, NNE, WestRock, Amcor, Constantia Flexibles - Hospitals and Pharmacies: Hospitals and Pharmacies Distribution: Hospitals and Pharmacies. To help customers receive essential neurointerventional equipment as quickly as possible.
Key Players: Medtronic, Stryker, Penumbra, Terumo, Cardinal Health, McKesson
Neurointerventional Devices Market Companies
- Medtronic: Top manufacturer of Solitaire stent removers, Pipeline flow siphons, and total access catheters to assist stroke and aneurysm therapies.
- Johnson and Johnson Services Inc.: Using Cerenovus, provides EmboTrap revascularization systems, embolization coils, and sophisticated catheter of the brain.
- Penumbra Inc.: Supplies ischemic stroke and aneurysm treatment with aspiration thrombectomy systems, RED catheters, and embolization coils.
Other Major Key Players
- Microport Scientific Corporation
- Stryker
- MicroVention Inc (Terumo Corporation)
- Codman Neuro (Integra Lifesciences)
Recent Developments
- In January 2026, Kaneka Medical America LLC announced the U.S. launch of the WaveSelectTM 1014 guidewire through an exclusive agreement with Enlight Medical Ltd. Utilizing proprietary polymer-metal matrix technology, WaveSelect enhances performance and deliverability for diverse neurovascular therapies, addressing key clinical needs. (Source: https://www.globenewswire.com )
- In July 2025, Q'Apel Medical received FDA clearance for its Zebra neurovascular access system, preparing for a full US launch. Available in 6Fr and 7Fr sizes, Zebra facilitates the introduction of interventional devices into peripheral and neurovasculature, enhancing compatibility and versatility for complex neurovascular procedures, according to a Q'Apel press release. (Source: https://neuronewsinternational.com )
- In June 2023, a leading company that makes medical devices known as BIOTRONIK has announced its launch of one solution, a multifunctional peripheral catheter called Oscar.
- In March 2023, a renowned American company called Johnson and Johnson presented innovative CEREPAK detachable coils in the U.S. These coils offer multiple coil sizes with three shapes dedicated to achieving concentric brain aneurysm filling to deliver efficient outcomes of the surgery and mitigate risk for brain dysfunctionality.
Segments Covered in the Report
By Product
- Stents
- Embolic Coils
- Catheter
- Wires
- Others
By Indication
- Stroke
- Brain Aneurysm
- Others
By Application
- Hospitals
- Ambulatory Surgical Center (ASC)
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting