What is the Non-Invasive Cancer Diagnostics Market Size?
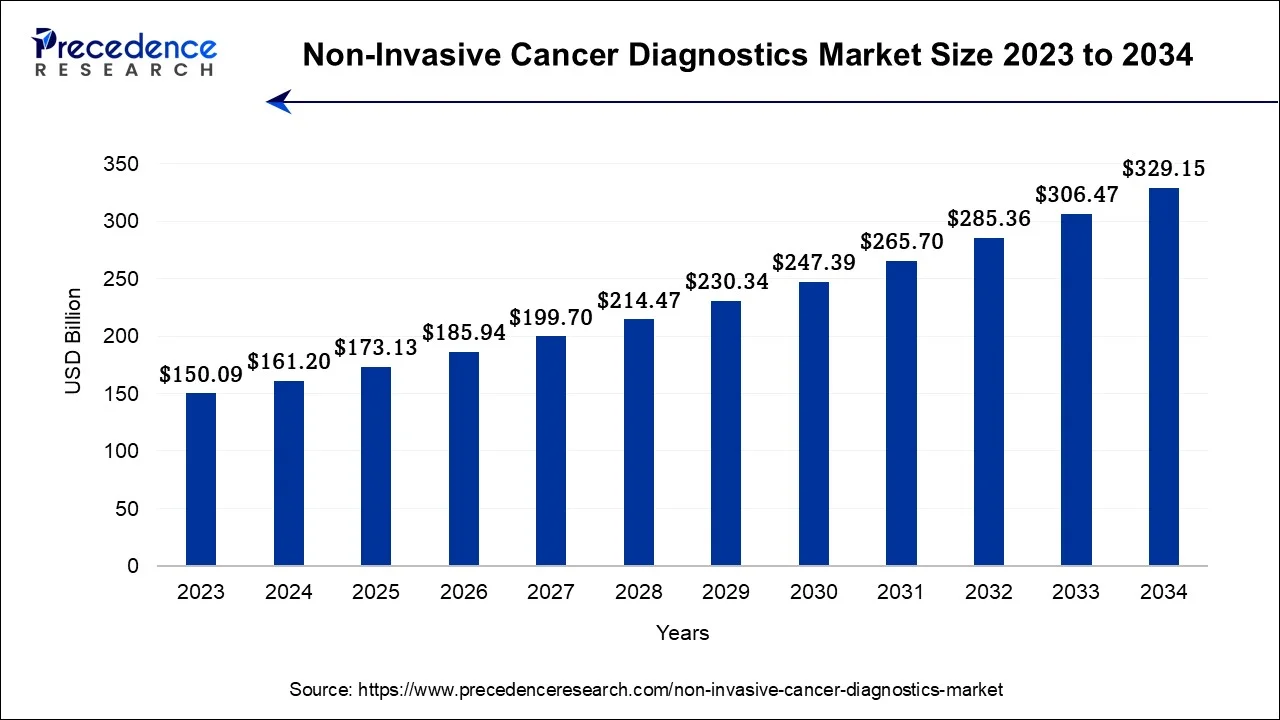
The global non-invasive cancer diagnostics market size was calculated at USD 173.13 billion in 2025 and is predicted to increase from USD 185.94 billion in 2026 to approximately USD 350.78 billion by 2035, expanding at a CAGR of 7.32% from 2026 to 2035.
Non-Invasive Cancer Diagnostics Market Key Takeaways
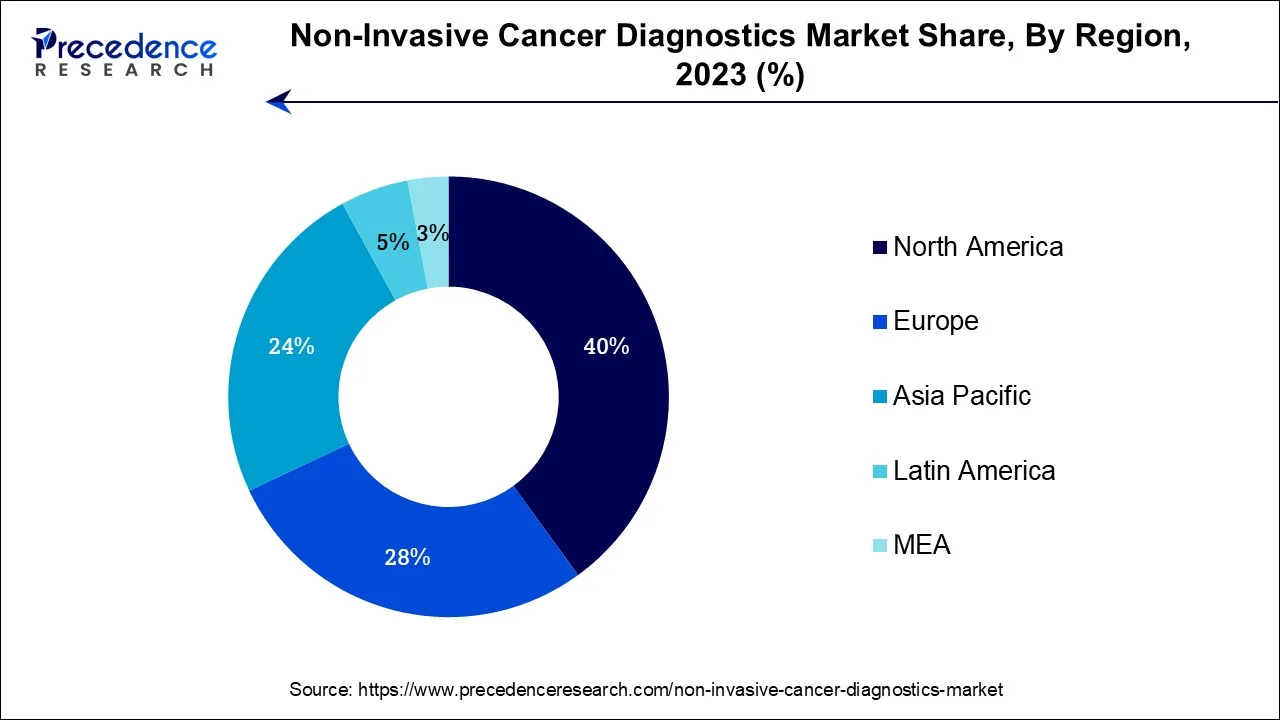
- North America held the highest revenue share 40% in 2025.
- Asia-Pacific is expected to expand at the fastest CAGR between 2026 and 2035.
- By Type, the breast cancer segment generated the largest market share of 16.3% in 2025.
- By Type, the curved display segment is anticipated to grow at a notable CAGR of 8.1% during the projected period.
- By Product Type, the clinical microbiology segment has held the maximum market share of 22% in 2025.
- By Product Type, the hematology segment is anticipated to grow at the fastest CAGR over the projected period.
- By Test Type, the imaging tests segment held the biggest revenue share of 22% in 2025.
- By Test Type, the spectroscopy segment is anticipated to expand at a remarkable CAGR of 10.9% over the predicted period.
- By Application, the blood segment contributed more than 45% of revenue share in 2025.
- By Application, the Saliva segment is estimated to grow at a noteworthy CAGR of 11.8% over the predicted period.
- By End User, the diagnostic centers segment captured over 35% of revenue share in 2025.
Market Overview
The realm of non-invasive cancer diagnostics unfolds as a burgeoning segment in the healthcare landscape, dedicated to the art of discerning malignancies without the need for intrusive procedures such as surgical biopsies. It encompasses an array of avant-garde technologies, encompassing liquid biopsy methodologies, advanced imaging modalities, and the meticulous scrutiny of blood-borne cancer biomarkers.
This domain's expansion is propelled by the surging demand for early-stage cancer detection, offering patients heightened comfort and rendering treatment strategies increasingly precise. Unfolding strides in genomics and molecular biology have paved the way for increasingly precise and individualized non-invasive diagnostic modalities, positioning this sector as a pivotal pillar in the global campaign against cancer.
Non-Invasive Cancer Diagnostics Market Growth Factors
The non-invasive cancer diagnostics market is experiencing a remarkable surge owing to its groundbreaking approach to detecting and monitoring cancer without invasive procedures like conventional biopsies. This dynamic domain encompasses an array of state-of-the-art technologies, encompassing liquid biopsies, advanced imaging methodologies, and the scrutiny of cancer-specific biomarkers in blood samples. The global non-invasive cancer diagnostics market has evolved rapidly, emerging as a pivotal component in the global crusade against cancer.
Numerous pivotal industry trends and growth catalysts are propelling the non-invasive cancer diagnostics market forward. Firstly, there is a burgeoning focus on early cancer detection and intervention, fueling demand for these non-invasive modalities. Secondly, advancements in genomics and molecular biology have ushered in more precise and personalized diagnostic approaches, elevating the market's allure. Thirdly, the diminishment of patient discomfort linked to non-invasive diagnostics is further expediting market expansion. Lastly, the escalating global incidence of cancer cases and the imperative for accessible and cost-effective diagnostic tools are steering the adoption of these innovative methods.
The market's growth is underpinned by a medley of growth drivers and offers a plethora of business prospects. The surging prevalence of cancer, particularly in aging demographics, provides a substantial customer base. Moreover, the emergence of innovative technologies and diagnostic platforms is fostering opportunities for innovation and market penetration. Collaborative ventures between healthcare institutions, research centers, and diagnostic firms can stimulate research and development endeavors, ultimately broadening the market's outreach and product portfolio. The global proliferation of telemedicine and the heightened demand for remote diagnostic solutions also present lucrative avenues for growth.
Despite its promising trajectory, the non-invasive cancer diagnostics market confronts significant challenges. Foremost among these are stringent regulatory examinations and approval processes for novel diagnostic technologies, which can be arduous and time-consuming. Additionally, the need for robust clinical validation and the standardization of non-invasive diagnostic techniques pose formidable hurdles. Market competition is fierce, with numerous contenders vying for market dominance, necessitating companies to distinguish themselves through ingenuity and quality. Lastly, reimbursement complexities and variations in healthcare infrastructure across different regions could impede the market's expansion.
In summation, the non-invasive cancer diagnostics market is poised for substantial expansion, propelled by trends accentuating early cancer detection, breakthroughs in genomics, and the quest for more patient-centric diagnostic solutions. While offering promising business opportunities, surmounting regulatory obstacles, ensuring rigorous clinical validation, and navigating competitive landscapes remain pivotal challenges for industry stakeholders aspiring to make a significant mark in this transformative domain.
Market Trends
- Rapid Growth of Liquid Biopsy Technologies: Liquid biopsies are gaining strong adoption as they enable cancer detection and monitoring through blood-based biomarkers such as ctDNA, CTCs, and exosomes. They support real-time disease tracking, early detection, and treatment response assessment without the need for invasive tissue biopsies.
- Integration of Artificial Intelligence and Digital Diagnostics:AI and machine learning are increasingly used to analyze imaging, pathology, and molecular data, improving diagnostic accuracy and speed. These technologies help detect subtle patterns, reduce human error, and support personalized treatment decisions.
- Expansion of Multi-Cancer Early Detection (MCED) Testing: MCED tests are emerging as a breakthrough by enabling simultaneous screening for multiple cancer types from a single blood sample. This trend is driving earlier diagnosis, especially for cancers that are difficult to detect with traditional screening methods.
Market Scope
| Report Coverage | Details |
| Growth Rate from 2026 to 2035 | CAGR of 7.32% |
| Market Size in 2025 | USD 173.13 Billion |
| Market Size in 2026 | USD 185.94 Billion |
| Market Size by 2035 | USD 350.78 Billion |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | By Type, By Product Type, By Test Type, By Application, and By End Users |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Non-invasive cancer diagnostics
The relentless surge in global cancer cases is a potent catalyst propelling the expansion of the non-invasive cancer diagnostics market. This continuous upswing in cancer occurrences can be ascribed to a myriad of factors, encompassing shifting demographics, evolving lifestyle patterns, environmental influences, and inherent genetic predispositions. As the shadow of cancer looms larger, there is an escalating demand for diagnostic techniques that are both efficacious and minimally intrusive.
Non-invasive cancer diagnostics emerge as a pivotal solution, providing the means to detect malignancies without subjecting individuals to the discomfort and potential hazards associated with invasive procedures such as surgical biopsies. Furthermore, the substantial economic burden borne by healthcare systems and societies due to the cancer challenge underscores the urgency for early and precise diagnostic tools.
Non-invasive modalities facilitate early detection, potentially leading to expedited interventions, improved treatment outcomes, and a potential reduction in the overall financial outlay linked to cancer care. Hence, the mounting incidence of cancer stands as a formidable driving force, energizing the non-invasive cancer diagnostics market as it endeavors to confront and mitigate the mounting healthcare challenge posed by cancer.
Restraints
Clinical validation
Clinical validation stands as a substantial impediment to the advancement of the non-invasive cancer diagnostics market. Ensuring the precision, dependability, and clinical credibility of non-invasive diagnostic techniques is a pivotal challenge. Prior to widespread adoption, these approaches must undergo extensive testing and validation procedures to establish their effectiveness in detecting cancer. This necessitates comprehensive clinical trials, meticulous data accumulation, and validation studies, all of which demand considerable resources and time.
The protracted validation process has the potential to delay the market entry of novel diagnostic technologies, constraining innovation and decelerating market growth. Furthermore, there exists a risk that certain non-invasive diagnostic methods may not meet the requisite standards of clinical accuracy, leading to skepticism among healthcare providers and potential patients. Consequently, the imperative for robust clinical validation, though vital to ensure patient safety and diagnostic reliability, can obstruct the swift expansion of the non-invasive cancer diagnostics market, creating impediments for emerging technologies striving to establish credibility and acceptance within the medical community.
Opportunities
Early detection emphasis
The increasing focus on early cancer detection stands as a pivotal driver of opportunities within the non-invasive cancer diagnostics market. As healthcare systems and individuals increasingly appreciate the profound advantages of identifying cancer in its earliest and most treatable stages, the demand for non-invasive diagnostic technologies is poised for remarkable expansion. This accentuation of early detection is fostering an environment of heightened research and inventive solutions in the field, nurturing the creation of state-of-the-art diagnostic tools capable of pinpointing cancer-related biomarkers, genetic anomalies, and other indicators with exceptional precision.
Such innovations empower healthcare providers to deliver timely interventions, thereby elevating patient outcomes and potentially reducing the overall financial burden of cancer care. Furthermore, this heightened awareness of early detection's benefits is stimulating patient engagement and fueling the demand for regular cancer screenings, thereby broadening the market's horizons. Consequently, the non-invasive cancer diagnostics market is expected to witness groundbreaking advancements and investment within the healthcare domain, all rooted in the burgeoning emphasis on the early detection of cancer.
Impact of COVID-19
The COVID-19 pandemic had a multifaceted impact on the non-invasive cancer diagnostics market. Initially, the market experienced disruptions due to strained healthcare resources and the postponement of elective procedures. However, as the pandemic highlighted the importance of remote and non-invasive diagnostic solutions, there was a subsequent surge in demand for telemedicine and non-invasive cancer diagnostic tools. The pandemic accelerated the adoption of these technologies, promoting innovation and market growth. Additionally, the emphasis on early detection of cancer gained prominence, further driving the market as healthcare systems sought more accessible and safer diagnostic options in the wake of the pandemic.
Segment Insights
Type Insights
The breast cancer sector held a 16.3% revenue share in 2025. The dominance of thebreast cancer segment within the non-invasive cancer diagnostics market can be attributed to several pivotal factors. Breast cancer ranks among the most prevalent malignancies worldwide, and its early detection is of paramount importance for enhanced treatment outcomes. Non-invasive diagnostic modalities, including breast MRI, mammography, and blood-based biomarker assessments, offer efficacious and minimally intrusive avenues for breast cancer detection.
The heightened awareness, dedicated screening initiatives, and relentless research efforts focused on breast cancer diagnostics have bolstered the demand for non-invasive solutions. Consequently, the breast cancer segment secures a significant share, reflecting the substantial demand and market potential inherent in breast cancer detection and surveillance.
The Curved Display sector is anticipated to expand at a significantly CAGR of 8.1% during the projected period. Lung cancer, renowned as one of the most prevalent and deadliest malignancies globally, has fostered an escalated demand for highly effective diagnostic solutions. Non-invasive methodologies, encompassing state-of-the-art imaging technologies, liquid biopsy methods, and cutting-edge diagnostic techniques, have demonstrated their exceptional value in the early identification of lung cancer, given the associated risks tied to more invasive alternatives. Furthermore, the relentless drive towards innovation and research has birthed sophisticated non-invasive tests that elevate diagnostic precision, further cementing the lung cancer segment's preeminence and dominant market growth.
Product Type Insights
Based on the product type, clinical microbiology is anticipated to hold the largest market share of 22% in 2025. The clinical microbiology segment commands a substantial share in the non-invasive cancer diagnostics market due to its pivotal role in diagnosing cancer through non-invasive means. This segment encompasses a wide range of diagnostic methods, including liquid biopsies and molecular assays, which analyze genetic and molecular markers present in bodily fluids, offering highly sensitive and specific cancer detection. The precision, convenience, and minimal discomfort associated with clinical microbiology-based non-invasive diagnostics have propelled its adoption, making it a cornerstone in early cancer detection and monitoring, thereby securing a significant market share.
The hematology segment is projected to grow at the fastest rate over the projected period. The Hematology division exerts substantial sway within the non-invasive cancer diagnostics arena owing to its central role in unearthing hematological malignancies like leukemia, lymphoma, and myeloma. Non-intrusive methodologies, encompassing approaches such as liquid biopsies and the scrutiny of blood-based biomarkers, have inaugurated a transformative era in the diagnosis of hematological cancers. They furnish the capacity for early detection and the continual tracking of maladies without necessitating intrusive procedures.
The efficacy of these methods, coupled with the mitigation of patient discomfort and their expanded range of applications, has cemented the Hematology segment's eminence as a pivotal contributor to market hegemony. As healthcare systems increasingly give precedence to the early identification and oversight of hematological cancers, this segment retains a substantial growth within the market.
Test Type Insights
The imaging tests segment held the largest revenue share of 22% in 2025. The imaging tests segment holds a substantial share in the non-invasive cancer diagnostics market primarily due to its established effectiveness in detecting and visualizing cancerous lesions and abnormalities. Modalities such as MRI, CT scans, and ultrasound provide detailed anatomical and functional information, aiding in accurate cancer diagnosis and staging.
Additionally, they offer non-invasive options for monitoring disease progression and treatment efficacy. Their widespread availability, proven track record, and patient-friendly nature contribute to their dominance in the market, making imaging tests a preferred choice for both clinicians and patients in the realm of non-invasive cancer diagnostics.
The spectroscopy segment is anticipated to grow at a significantly faster rate, registering a CAGR of 10.9% over the predicted period. The spectroscopy segment commands a significant growth in the non-invasive cancer diagnostics market due to its ability to offer precise and non-invasive insights into cancer at the molecular level. Spectroscopy techniques, such as Raman and infrared spectroscopy, enable the identification of biomarkers and abnormal tissue characteristics without invasive procedures.
This technology's accuracy and versatility in detecting various cancer types have propelled its adoption. Additionally, advancements in spectroscopic imaging and data analytics have enhanced diagnostic capabilities, further solidifying its position as a preferred choice for non-invasive cancer diagnostics, leading to major market growth.
Application Insights
The blood segment has generated revenue share of 45% in 2025. The prominence of the blood segment within the non-invasive cancer diagnostics market is attributed to its efficacy in detecting cancer-related biomarkers and genetic material. Blood-based assessments, such as liquid biopsies, present a minimally intrusive means of cancer screening and monitoring, rendering them more attractive to both patients and healthcare providers.
Furthermore, advancements in the realms of molecular biology and genomics have elevated the precision of these tests, bolstering their adoption. Offering the potential to identify various cancer types, blood-based diagnostics have emerged as a versatile and dependable asset in the battle against cancer, contributing significantly to their substantial market presence.
The Saliva segment is anticipated to grow at a significantly faster rate, registering a CAGR of 11.8% over the predicted period. The dominance growth of the Saliva sector within the non-invasive cancer diagnostics market can be attributed to its notable advantages, characterized by its ease of use, patient-friendly approach, and wide accessibility. Saliva-based diagnostics have gained prominence due to their non-intrusive collection method, simplifying the sampling process while eliminating the necessity for invasive procedures like tissue biopsies.
Furthermore, advancements in molecular biology have expanded the horizon for detecting cancer biomarkers in saliva, enhancing its diagnostic potential. With this combination of factors and a growing focus on research and development, the Saliva segment has established a significant foothold in the market, emerging as a prominent growth leader to the quest for effective and patient-centered cancer diagnostic techniques.
End User Insights
The diagnostic centers segment has generated a revenue share of 35 % in 2025. The diagnostic centers segment holds a significant share in the non-invasive cancer diagnostics market due to its specialized expertise, advanced equipment, and comprehensive diagnostic services. Diagnostic centers are well-equipped to perform a wide range of non-invasive cancer diagnostic tests efficiently. They often collaborate with healthcare providers, offering convenient access to these services for patients.
Moreover, diagnostic centers typically stay updated with the latest technologies, ensuring accurate and reliable results. This reliability and convenience make them a preferred choice for both healthcare professionals and patients, contributing to their dominant position in the market.
The ambulatory care segment is anticipated to grow at a significantly faster rate, registering a CAGR of 8.6% over the projected period. The ambulatory care segment holds substantial growth in the non-invasive cancer diagnostics market due to its patient-centric advantages. It offers the convenience of non-invasive diagnostic procedures performed outside traditional hospital settings, reducing patient discomfort and healthcare costs.
Additionally, the rising emphasis on early cancer detection has led to increased outpatient screenings and diagnostics. Ambulatory care facilities are well-suited for these purposes, making them a preferred choice. Moreover, the COVID-19 pandemic accelerated the adoption of telemedicine, further bolstering the ambulatory care segment, as it aligns with remote and non-invasive diagnostic trends.
Regional Insights
U.S. Non-Invasive Cancer Diagnostics Market Size and Growth 2026 to 2035
The U.S. non-invasive cancer diagnostics market size was accounted at USD 48.48 billion in 2025 and is expected to be worth around USD 100.64 billion by 2035, at a CAGR of 7.58% from 2026 to 2035.
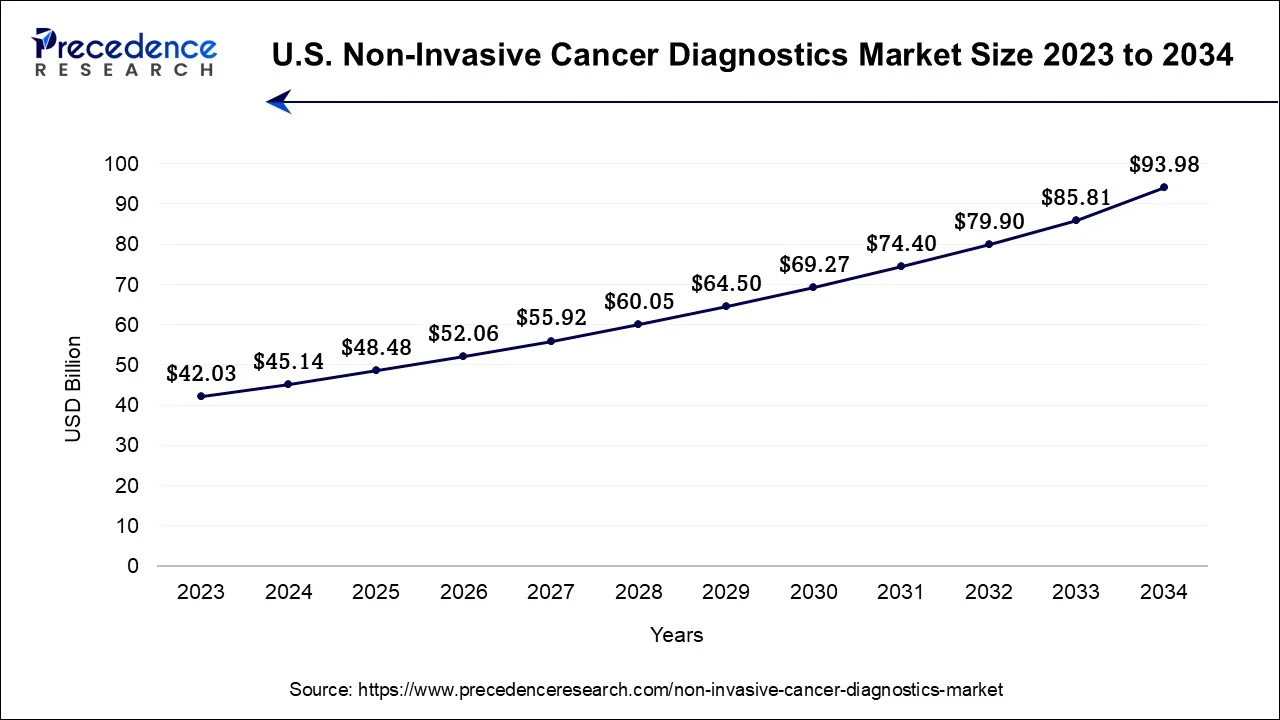
North America has held largest revenue share 40% in 2025. North America commands a significant share of the non-invasive cancer diagnostics market due to several factors. The region boasts advanced healthcare infrastructure, a strong focus on early cancer detection, and a high prevalence of cancer cases. Robust research and development activities, coupled with significant investments in cutting-edge diagnostic technologies, contribute to the region's dominance.
Moreover, favorable reimbursement policies, a well-established healthcare regulatory framework, and a proactive approach to healthcare innovation further drive market growth. Additionally, the region's willingness to adopt new technologies and the presence of key market players solidify North America's prominent position in this dynamic industry.
U.S. Market Analysis
The U.S. dominates the North American market due to its high cancer incidence rates, robust clinical research ecosystem, and early commercialization of novel diagnostic technologies. There is high use of liquid biopsy tests, genomic profiling, and non-invasive imaging solutions, driven by precision medicine initiatives. Favorable regulatory pathways, strong presence of leading diagnostic companies, and increasing adoption of at-home and blood-based cancer screening tests further accelerate market expansion.
What Makes Asia Pacific the Fastest-Growing Market for Non-Invasive Cancer Diagnostics?
Asia-Pacific is estimated to observe the fastest expansion. The Asia-Pacific region commands a significant share of the non-invasive cancer diagnostics market due to several key factors. Firstly, the region's large and aging population contributes to a higher incidence of cancer cases, boosting demand for diagnostic tools. Secondly, increasing healthcare expenditure and improving healthcare infrastructure have enhanced accessibility to non-invasive cancer diagnostics. Thirdly, a growing awareness of the importance of early cancer detection has driven adoption. Lastly, the presence of prominent market players and ongoing technological advancements in countries like China, Japan, and India has propelled the region's prominence in this market.
India Market Analysis
India represents a high-growth market due to increasing cancer burden and growing demand for cost-effective non-invasive testing solutions. Adoption of blood-based biomarkers, imaging diagnostics, and AI-enabled screening tools is rising, particularly in urban centers. Government initiatives to expand cancer screening, coupled with the growth of private diagnostic chains and medical tourism, are supporting market expansion.
What Potentiates the Market in Europe?
The market in Europe is expected to grow steadily during the forecast period, supported by government-funded cancer screening programs, rising emphasis on early diagnosis, and strong regulatory focus on preventive healthcare. The region has witnessed increasing adoption of non-invasive diagnostic modalities such as biomarker-based blood tests, advanced imaging, and molecular diagnostics. Cross-border research collaborations, growing elderly populations, and expanding access to cancer screening services contribute to market development.
Germany Market Analysis
Germany plays a leading role in the European market due to its well-established healthcare system, strong diagnostics manufacturing base, and high adoption of advanced medical technologies. The country actively integrates non-invasive cancer screening tools within hospital and outpatient settings. Strong reimbursement coverage, emphasis on early cancer detection, and continuous investments in oncology research and digital diagnostics support consistent market growth.
Non-Invasive Cancer Diagnostics Market Companies
- Abbott
- Thermo Fisher Scientific, Inc.
- Illumina, Inc.
- QIAGEN
- F. Hoffmann-La Roche Ltd
- Agilent Technologies, Inc.
- Quest Diagnostics Incorporated.
- Merck KGaA
- Hologic, Inc.
- BD.
- GSK plc.
- Novartis AG
- Bristol-Myers Squibb Company
- Lilly.
- Pfizer, Inc.
- Myriad Genetics, Inc.
Recent Developments
- In December 2025, ErlySign's oral cancer detection kit is expected to launch commercially by April 2026, following the completion of large-scale clinical trials in December 2025. The company is currently seeking regulatory approval and increasing manufacturing capacity. (Source: biospectrumindia.com)
- In July 2025, Compremium, a Swiss medtech company, began its initial human clinical study for a new non-invasive device for diagnosing thyroid cancer, which remains in progress as of January 2026. (Source: ggba.swiss)
- In July 2025, Gnosis launched EdenDx, marking the first commercially available non-invasive liquid-based cytology test in the U.S. for detecting early-stage endometrial cancer. (Source: insideprecisionmedicine.com)
- In 2023, Roche announced the expansion of its collaborative efforts with Janssen, focusing on advancing personalized healthcare through companion diagnostics. This partnership aims to further tailor medical treatments to individual patients, marking a significant stride in the evolution of healthcare delivery.
- In 2022,RadNet, Inc.'s joint venture, New Jersey Imaging Network (NJIN), completed the acquisition of Montclair Radiology's outpatient radiology assets, comprising six imaging centers in northern New Jersey. This strategic move solidifies RadNet's position as a prominent provider of outpatient imaging services in the northern and central regions of New Jersey. The primary goal is to offer cost-effective, high-quality, and convenient imaging solutions as a more affordable alternative to hospital-based options.
- In 2022, Unilabs Sweden entered into a partnership with Subtle Medical, a healthcare technology company specializing in leveraging artificial intelligence to enhance MRI imaging. This collaboration aims to elevate the efficiency and quality of MRI scans. Subtle Medical's innovative SubtleMR software aids radiologists in producing high-quality images while significantly reducing scan times by more than 60%. Notably, this software has received clinical validation through peer-reviewed journals, demonstrating its effectiveness and reliability across various healthcare settings, scanner types, and patient populations.
Segments Covered in the Report
By Type
- Lung Cancer
- Breast Cancer
- Solid Tumors
- Blood Cancer
- Ovarian Cancer
- Colorectal Cancer
- Other
By Product Type
- Immunochemistry
- Clinical Microbiology
- Point of Care Test (POCT)
- Hematology
- Hemostasis)
By Test Type
- Urine Test
- Imaging Test
- Computerized Tomography
- Magnetic Resonance Imaging
- Nuclear Medicine Scans
- X-ray/Mammography
- Ultrasound
- Spectroscopy
By Application
- Blood
- Urine
- Saliva
By End Users
- Hospital and Clinics
- Diagnostic Centers
- Ambulatory care
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting