Photodynamic Therapy Market Size and Forecast 2025 to 2034
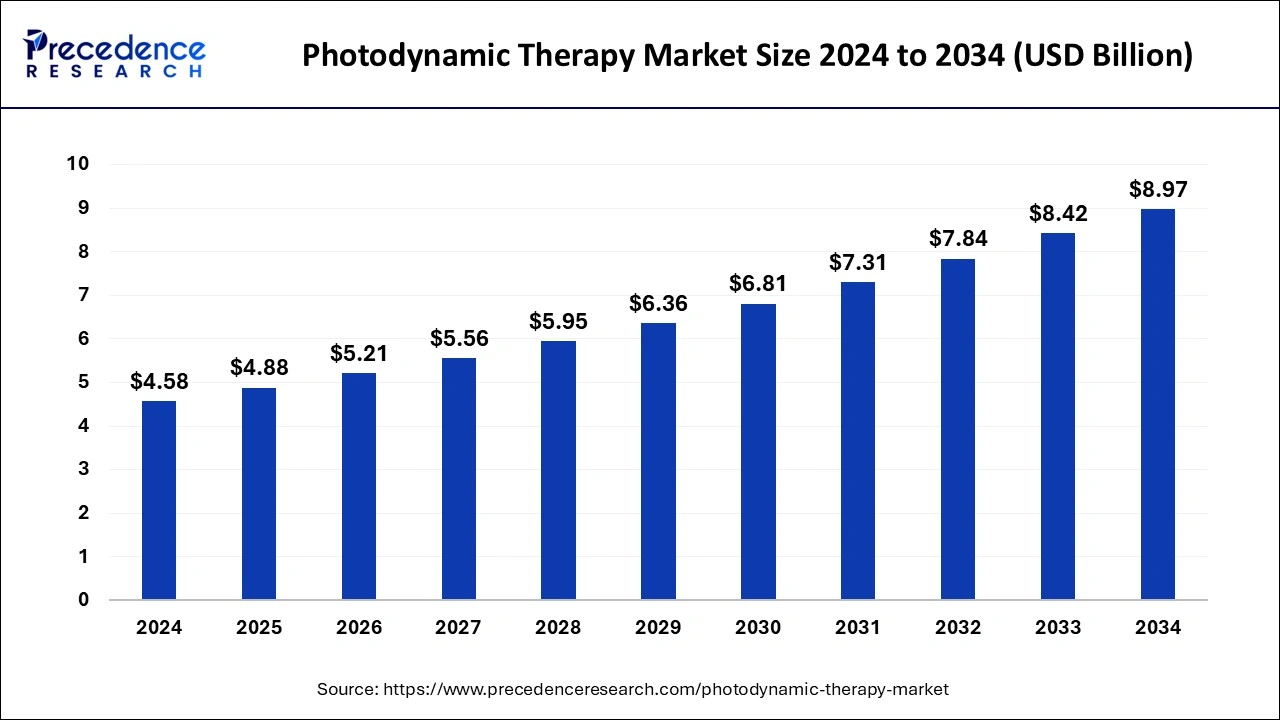
The global photodynamic therapy market size was estimated at USD 4.58 billion in 2024 and is predicted to increase from USD 4.88 billion in 2025 to approximately USD 8.97 billion by 2034, expanding at a CAGR of 6.95% from 2025 to 2034.
Photodynamic Therapy MarketKey Takeaways
- The global photodynamic therapy market was valued at USD 4.58 billion in 2024.
- It is projected to reach USD 8.97 billion by 2034.
- The market is expected to grow at a CAGR of 6.95% from 2025 to 2034.
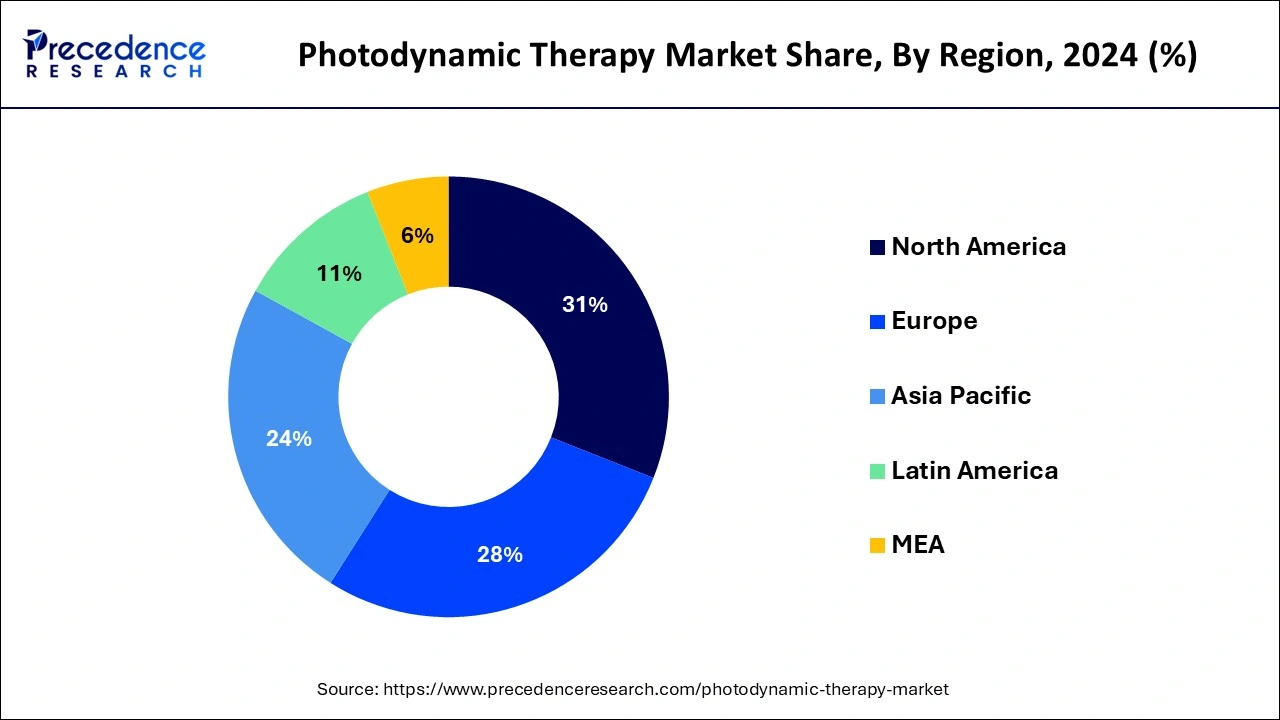
- North America dominated the photodynamic therapy market with the largest share of 31% in 2024.
- By product type, the photosensitizers drugs segment held the largest share of the market in 2024.
- By light source, the lasers segment dominated the market in 2024.
- By application, the cancer segment has the largest share in the photodynamic therapy market in 2023.
- By end-user, the hospitals segment dominated the market in 2024.
U.S.Photodynamic Therapy Market Size and Growth 2025 to 2034
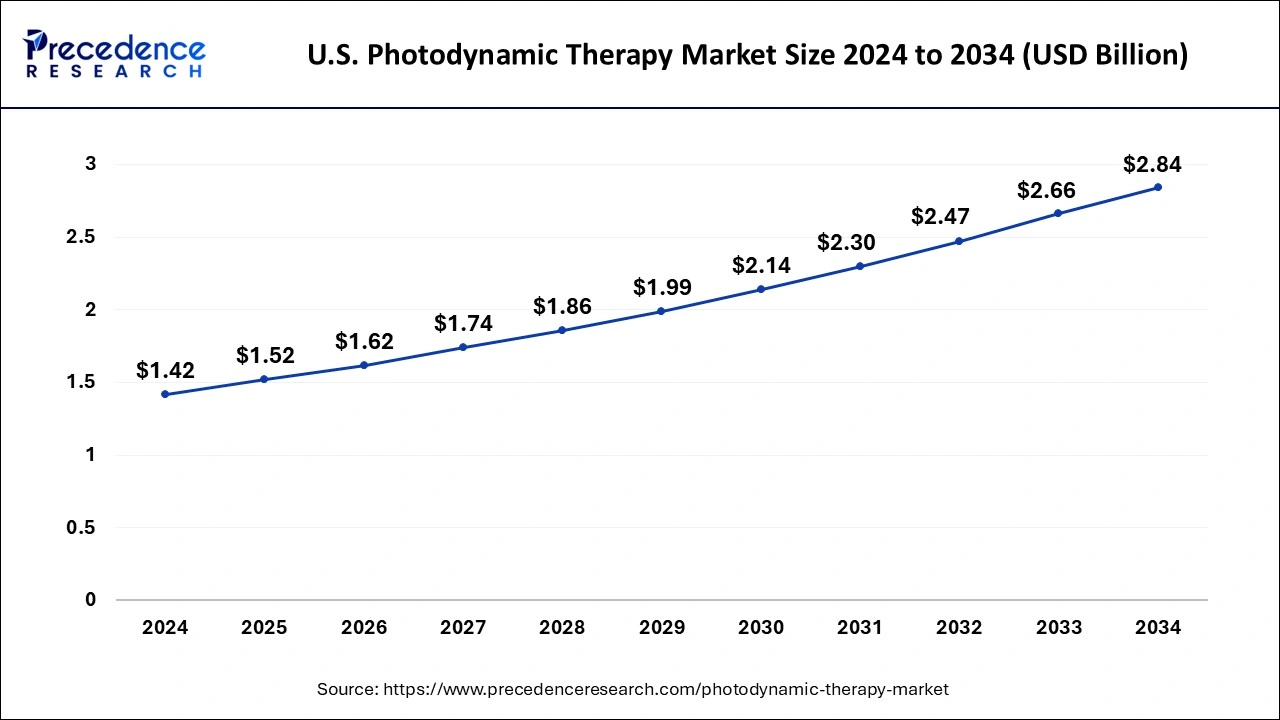
The U.S. photodynamic therapy market size was valued at USD 1.42 billion in 2024 and is expected to reach around USD 2.84 billion by 2034, growing at a CAGR of 7.18% from 2025 to 2034.
North America held the largest market share of 31% in 2024. Prominent biotech and pharmaceutical businesses are widely distributed throughout North America, especially in the United States. These companies make significant R&D investments, which enables them to create and market cutting-edge PDT technologies. Regulatory organizations like the food and drug administration (FDA) in the US greatly aid medical technology approval and regulation in North America. A practical and supportive regulatory framework can facilitate PDT product introduction.
Asia-Pacific is observed to witness the fastest growth during the forecast period of 2025-2034. Skin-related illnesses, including skin cancer and dermatological diseases, are prevalent in this area. PDT is frequently applied to various ailments. The public and medical experts are becoming increasingly aware of the advantages of photodynamic treatment. PDT will become more widely used as a therapeutic option as knowledge increases. Growing disposable income in many Asia-Pacific nations enables people to choose more sophisticated, frequently more costly medical procedures, supporting the PDT industry's expansion.
- For instance, in December 2023, researchers from the University of Hong Kong's LKS Faculty of Medicine (HKUMed) and partners at Sun Yat-sen University's Zhongshan Ophthalmic Centre in Guangzhou created a light-activatable prodrug nanotechnology in medicine for the treatment of age-related macular degeneration (AMD). Anti-angiogenic and photodynamic therapy in combination can be triggered by injecting nanomedicine intravenously and irradiating diseased eyes with light. This minimally invasive approach can be used to treat AMD and other ocular disorders categorized by aberrant blood vessel growth.
Market Overview
Photodynamic therapy (PDT) is frequently used in oncology to treat bladder, esophagus, lung, and skin malignancies. After the photosensitizing chemical is applied, reactive oxygen species that destroy cancer cells when they encounter light are formed. It is used to treat various skin disorders, including actinic keratosis, acne, and some forms of skin cancer. It provides a non-invasive substitute for conventional procedures, including surgery. Dental professionals can use PDT to treat periodontal disorders. When triggered by light, it aids in the removal of germs and diseased gum tissue. Additionally, it is used in veterinary medicine to treat animal cancers. In some situations, it offers a less invasive alternative to standard surgery.
- In July 2023, according to a statement from the medicine's inventor, ImPact Biotech was given orphan drug status by the FDA for use as a possible therapeutic option for individuals with locally advanced pancreatic cancer. The product comprises the photosensitizer padeliporfin, administered intravenously through a laser light delivery system. Tumor necrosis is caused by boosting antitumor immunity and intensifying cancer cell destruction.
- In February 2023, The FDA gave Elranatamab's biologics license application top priority to treat people who have relapsed or refractory multiple myeloma (RRMM).
Photodynamic Therapy Market Growth Factors
- Globally rising cancer rates, especially those of the skin, are driving up demand for complementary therapies like photodynamic therapy.
- When compared to conventional therapies like surgery, radiation, and chemotherapy, PDT is preferred by patients because of its less invasive nature and lower risk of side effects.
- PDT's commercial reach is increased by its adaptability in treating a range of malignancies, skin disorders, and other illnesses.
- Advancements in light sources, photosensitizers, and delivery systems enhance PDT's efficacy, accuracy, and user-friendliness.
- Market expansion is facilitated by regulatory agencies' approval of innovative photosensitizers and gadgets.
- Spending on healthcare is growing globally, which opens the potential for PDT and other cutting-edge technologies.
Market Scope
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 6.95% |
| Market Size in 2025 | USD 4.88 Billion |
| Market Size by 2034 | USD 8.97 Billion |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Product Type, By Light Source, By Application, and By End User |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increasing government support for research and development
Globally, governments acknowledge PDT's potential as a cutting-edge and promising medical treatment, especially in cancer treatment. Government support through financial resources and encouragement helps push PDT technologies forward, stimulate new discoveries, and refine current practices.
Government assistance for research studies to create and improve PDT techniques can take many forms, including grants, subsidies, and funding. With the help of this funding, businesses and researchers can perform clinical trials, invest in state-of-the-art technologies, and investigate novel applications for PDT that go beyond its current use.
Regulatory agencies and research organizations frequently work together to develop standards and guidelines for using PDT safely and efficiently. This regulatory framework encourages investment and innovation by helping to establish a favorable environment for industry participants.
Growing public awareness of PDT
PDT is a medical procedure that uses light and photosensitizing chemicals to treat several illnesses, such as cancer and some skin problems. The demand for PDT therapy rises as knowledge grows and more people learn about the advantages and uses of this treatment technique. Patient preferences and choices for medical treatments are greatly influenced by public awareness. This factor is observed to act as a driver for the photodynamic therapy market.
With increasing knowledge of photodynamic therapy's effectiveness, low invasiveness, and potential benefits over conventional therapies, individuals tend to choose PDT. The market for photodynamic treatment is growing due to this greater awareness, raising interest from academics, investors, and healthcare professionals and increasing patient acceptance rates.
Restraint
Complex regulatory landscape
PDT products are subject to regulatory approval only after extensive clinical trials proving their safety and effectiveness. Preclinical research, investigational new drug (IND) uses, and lengthy human clinical trials are just a few of the stages that make up the process. The protracted approval process may make it more difficult for PDT products to be released on schedule. Variations in treatment factors, including light sources, photosensitive substances, and dosimetry, make standardizing PDT methods difficult.
A variety of PDT treatments, achieving uniformity and repeatability in clinical outcomes is required by regulatory bodies and may take some time. Distinct nations and areas have distinct PDT regulatory environments. Businesses that conduct business internationally must manage a patchwork of laws that demand adherence to various norms and rules.
Opportunities
Expanding range of applications
PDT is still a promising treatment for several kinds of cancer. Enhancing its market potential is extending its application to various cancer types and stages. The treatment of eye conditions such as age-related macular degeneration (AMD) is being investigated with it. Increasing its application to ocular problems may create new opportunities for market expansion. PDT can be more effective by customizing it to each patient's unique profile based on genetic, molecular, or other characteristics. This opens new possibilities for applications in personalized medicine.
Actinic keratosis, psoriasis, and acne are the skin disorders that PDT treats in dermatology. Its use in treating different dermatological disorders may rise as knowledge and study advance. It has demonstrated potential in treating pain, especially in the case of arthritic disorders. PDT may be used in more pain management procedures when this field of study develops.
Research on next-generation photosensitizers
Photodynamic therapy is a medical intervention that selectively destroys or damages target cells and tissues using light, oxygen, and photosensitizing chemicals. The efficiency of photosensitizers is a significant factor in PDT's success. Developing next-generation photosensitizers requires investigating novel chemicals, nanomaterials, and delivery techniques to improve the therapeutic results of photodynamic therapy. Researchers are working to increase photosensitizers' safety, efficacy, and selectivity, enhancing the possibilities of treating various illnesses, such as cancer and several dermatological disorders.
This research focuses on increasing photosensitizers' absorption and emission properties, enhancing their biocompatibility, and customizing their pharmacokinetics for improved targeting. Technological advancements in molecular design and nanotechnology are critical to developing photosensitizers that can overcome the drawbacks of existing PDT methods.
Product Type Insights
The photosensitizer drugs segment dominated the photodynamic therapy market in 2024. Photosensitizer drugs are made to selectively concentrate in target cells, guaranteeing a more accurate and focused course of treatment. This selectivity reduces the likelihood of harm to the adjacent, healthy tissues. The adoption of photosensitizer medications has increased due to the favorable patient outcomes and growing acceptance of photodynamic therapy in clinical practice. Photosensitizer medications are an essential part of cancer therapy since photodynamic therapy is widely utilized to treat a variety of malignancies. The popularity of photosensitizer-based photodynamic therapy has been greatly aided by its capacity to target and eliminate cancer cells accurately.
Light Source Insights
The lasers segment held the dominating share of the photodynamic therapy market in 2024. The accurate and controlled delivery of light energy by lasers is essential for activating photosensitizing photodynamic therapy (PDT) drugs. This accuracy effectively treats the targeted location while reducing injury to nearby healthy tissues. Because of their versatility, lasers are used in many different medical applications and can be utilized for various PDT procedures.
Compared to several other treatment modalities, laser-based photodynamic therapy (PDT) has a lower chance of side effects and problems because of its focused nature. Because of this, laser-based PDT is the better option for several medical issues.
Application Insights
The cancer segment held the largest market share in 2024and it is expected to grow significantly in the upcoming period. Globally, cancer is a significant and pervasive health hazard. The increasing prevalence of diverse forms of cancer has fueled the need for efficient and focused therapeutic approaches, such as photodynamic therapy.
In the treatment of certain cancers, photodynamic therapy has demonstrated encouraging outcomes. It uses photosensitizing chemicals triggered by light wavelengths to destroy cancer cells. The need for photodynamic therapy has grown as patients and medical professionals become more knowledgeable about cutting-edge and alternative cancer treatments. This is especially true for some cancer types where PDT has shown promise.
The acne segment is expected to grow at the fastest CAGR during the forecast period. By employing light to activate photosensitizing chemicals, photodynamic treatment provides a tailored method that targets and destroys sebaceous glands and germs that cause acne.
Alternatives to conventional acne remedies like topical creams and oral drugs, which may have adverse effects or be ineffective, are becoming increasingly popular among patients. PDT is a desirable alternative for people looking for efficient acne treatment because of its non-invasiveness, low risk of side effects, and ability to be customized depending on a patient's unique skin type.
End-user Insights
The hospitals segment held the largest share of the photodynamic therapy market in 2024. Hospitals offer a regulated and well-established clinical environment essential for administering photodynamic treatment. Hospital facilities and infrastructure are built to handle medical procedures, guaranteeing the efficacy and safety of PDT treatments. Specialized equipment, such as light sources and diagnostic tools, are needed for photodynamic therapy. Because hospitals usually have access to cutting-edge medical technology, PDT equipment is always available.
The cosmetic and dermatology clinics segment is observed to witness the fastest expansion during the forecast period. The need for cosmetic operations to improve appearance and manage skin issues is rising globally. PDT is provided by dermatology and cosmetic clinics as a non-invasive, successful treatment option for a range of skin disorders, drawing in a more extensive patient base that is looking for cosmetic remedies. Patient education has increased due to a fuller understanding of the advantages of PDT for skin rejuvenation and therapy. Clinics for dermatology and cosmetic surgery are vital for knowledge dissemination, which helps explain why PDT treatments are becoming increasingly popular while promoting the segment's growth in the photodynamic therapy market.
Photodynamic Therapy Market Companies
- Gladerma S.A.
- Sun Pharmaceutical Industries Ltd.
- Biofrontera
- Novartis AG
- Valeant Pharmaceuticals International, Inc.
- Quest Pharmatech, Inc.
- Hologic, Inc.
- Lumibird (Quantel Medical)
- Theralase Technologies Inc.
- Photocure ASA
- Biolitec AG
- IPG Photonics Corporation
Recent Developments
- In September 2022, Quest PharmaTech Inc. declared that the Photodynamic Therapy technology license would be terminated and returned, as well as the sale of its ownership stake in Bioceltran Co., Ltd. for $300,000. A private company established in Korea called Bioceltran is creating active molecules that penetrate the skin for use in cosmetic and medicinal applications.
Segments Covered in the Report
By Product Type
- Photosensitizer Drugs
- Photodynamic Therapy Devices
By Light Source
- Lasers
- Light Emitting Diodes (LEDs)
- Lamps
- Others
By Application
- Cancer
- Actinic Keratosis (AK)
- Psoriasis
- Acne
- Others
By End User
- Hospitals
- Cancer Treatment Centers
- Cosmetic and Dermatology Clinics
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content