What is the Preclinical CRO Market Size?
The global preclinical CRO market sizeis accounted at USD 6.76 billion in 2025 and predicted to increase from USD 7.29 billion in 2026 to approximately USD 13.14 billion by 2034, representing a CAGR of 7.66% from 2025 to 2034.
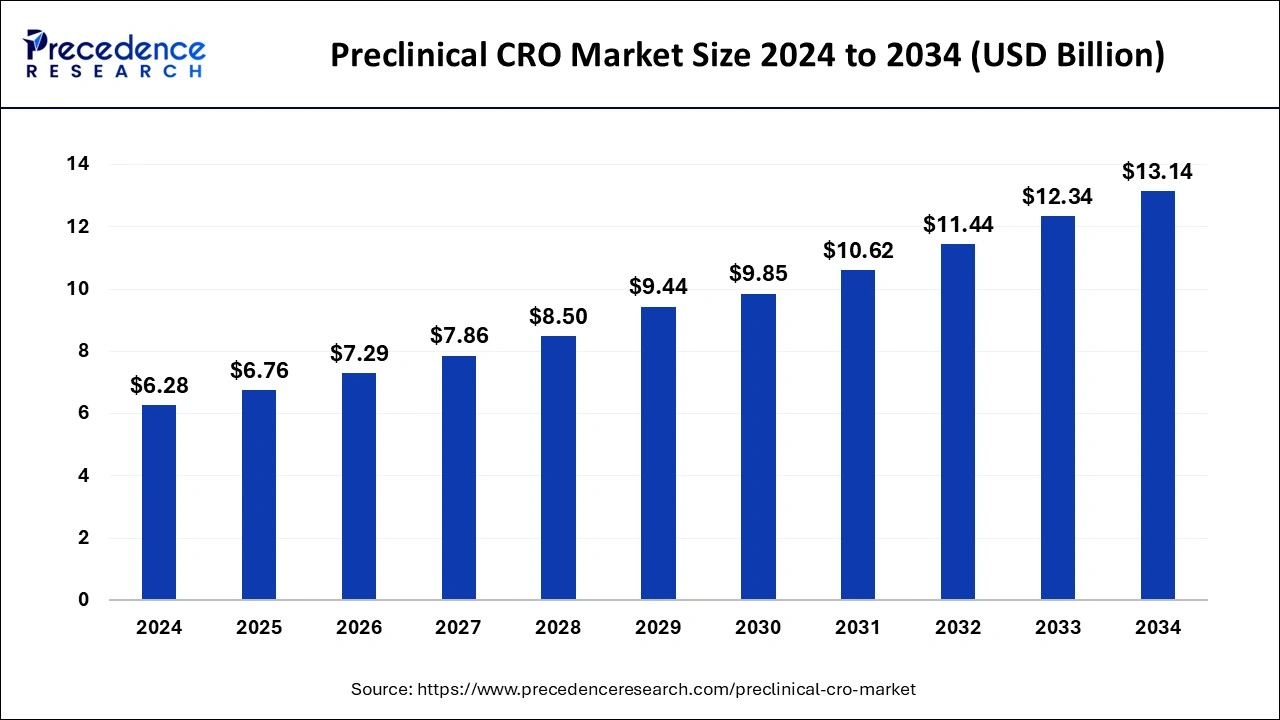
Preclinical CRO Market Key Takeaways
- North America led the global market with the highest market share of 47.14% in 2024.
- Asia Pacific region is expected to grow at a CAGR of 10.9% Over the forecast period 2025 to 2034.
- The bioanalysis and DMPK studies segment is poised to register a growth at a CAGR of 8.5% from 2025 to 2034.
- The government and academic institutes segment is expected to grow at a CAGR of 8.2% from 2025 to 2034.
AI in the Market
AI has a groundbreaking effect on preclinical and clinical research because of its vast advantages in terms of accuracy, speed, and proper judgment. It shortens the drug discovery process through predictive modeling, molecular simulation, and in silico testing. Automation makes AI-driven labs less occupied, more productive, and also helps in study design, toxicity prediction, and data interpretation. Moreover, it enhances compliance and documentation processes that guarantee regulatory adherence. Apart from that, AI is the one optimizing workflows, cutting costs, and increasing preclinical testing. Its merging with bioinformatics and digital platforms is facilitating the move of new medicines into the market and making preclinical operations more efficient.
Preclinical CRO Market Growth Factors
The rapid expansion and growth of the contract research organizations (CROs) in the past few years has boosted the growth of the preclinical CRO market. Various biopharmaceutical companies, medical device companies, and government research organizations avail the services provided by the CROs on a contract basis. The rising prevalence of various chronic diseases and gene-related diseases needs to be catered to. This need resulted in the increased investments in the research activities by the numerous biopharmaceutical and medical device companies, for the development of new and innovative drugs and devices for the diagnosis, prevention, and treatment of chronic diseases. Preclinical CROs enables the biopharmaceutical and medical device companies to focus on their core activities by providing the solutions and services regarding the research and development of the medicine and devices. Therefore, the rapid growth and development of the biopharmaceutical industry is expected to play a major role in the growth of the global preclinical CRO market.
The rising need for the development of new life-saving drugs is boosting the investments in the preclinical studies. Preclinical CROs are playing a crucial role in the development of new drugs and staging the concepts for the commercialization of the newly developed medicines. The recent outbreak of the COVID-19 virus has significantly contributed towards the increased activity of the preclinical CROs. The COVID-19 was new to the world, and hence its preclinical study was of utmost importance. This resulted various organizations and governments to inject huge investments in the preclinical research of the virus. Moreover, favorable government policies and regulations regarding the preclinical studies especially in the developed regions of North America and Europe are the major drivers of the preclinical CRO market across the globe.
- The increasing need for drug discovery and development services is driving the preclinical contract research organizations (CROs) market, allowing biopharmaceutical companies to hasten their research.
- The demand for chronic and genetic disease treatments is a major factor contributing to the rise in preclinical trials aimed at the development of cutting-edge therapies.
- The outsourcing of research activities to CROs allows pharmaceutical manufacturers and medical device makers to concentrate more on their primary areas of expertise, while at the same time reducing costs incurred in research and development.
- Bioengineering and toxicology research developments are expanding the range of preclinical studies.
Progressive government policies and more active partnerships between public and private research institutions are the main factors supporting the growth of the market in various parts of the world.
Market Scope
| Report Highlights | Details |
| Market Size in 2025 | USD 6.76 Billion |
| Market Size by 2034 | USD 13.14 Billion |
| Growth Rate from 2025 to 20344 | CAGR of 7.66% |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Service, and End User |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Service Insights
Based on service, the toxicology testing segment dominated for around 24% of the market share in 2024. As per the research results published by the Sevier Research Institute, around 50% of the preclinical testing witnessed failure owing to the toxicology tests. Therefore the extensive use of the toxicology testing across the preclinical CRO industry is anticipated to drive the demand for the preclinical CRO services.
On the other hand, the bioanalysis & DMPK studies segment is estimated to be the fastest-growing segment during the forecast period. This is attributed to the increased importance of the DMPK in evaluating the intrinsic properties of drugs and facilitates toxicology testing. Moreover, bioanalysis provides quantitative analysis and is increasing being used at every stage of medicine development process. Therefore the rising uses of bioanalysis and DMPK in the preclinical research is estimated to drive the growth of this segment.
End Use Insights
Based on end use, the biopharmaceutical segment accounted for around 82% of the market share in 2024. The increasing number of medium and small biopharmaceutical companies across the globe is boosting the growth of this segment. The small and medium-sized biopharmaceutical companies lack in adequate resources and expertise in preclinical trials of drug development. Therefore, they are increasingly outsourcing the preclinical research works to the preclinical CROs that boosts the demand for the preclinical CROs services. Further, the rapid growth of the biopharmaceutical industry is expected to drive the demand for the preclinical CRO services in the upcoming future. The biopharmaceutical industry alone accounts for around 20% of the global pharmaceutical industry and is rapidly growing.
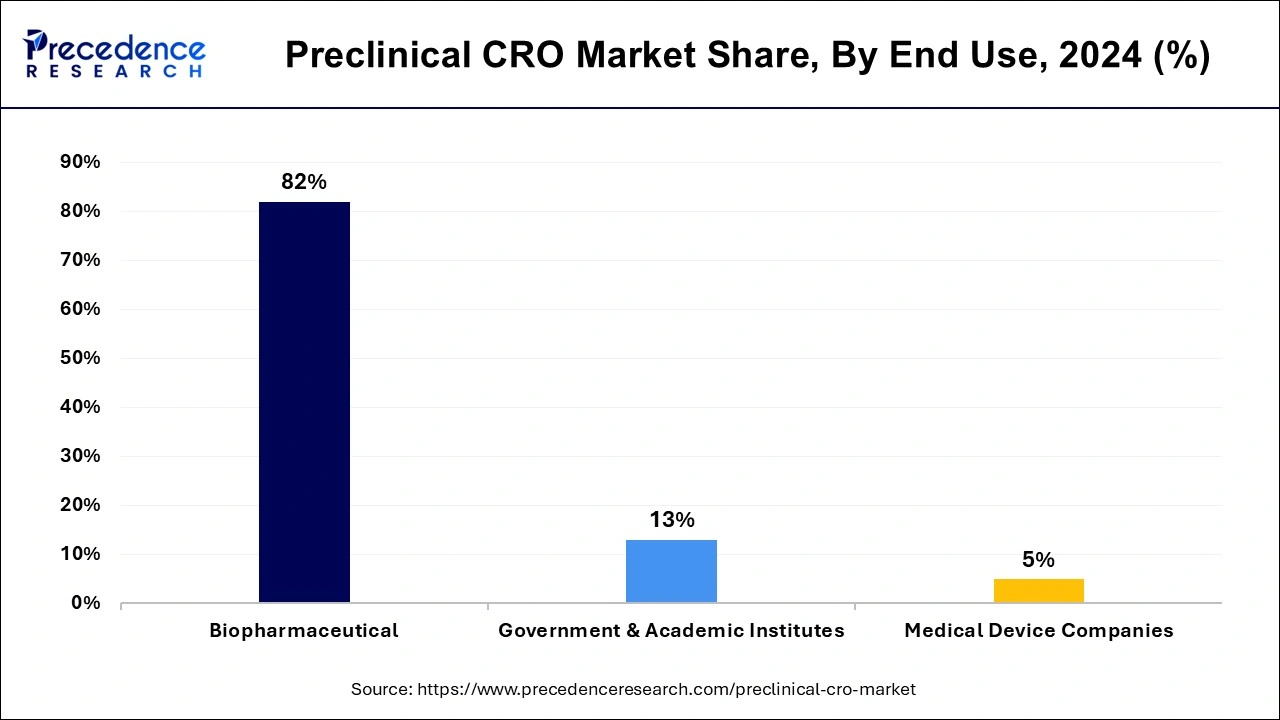
On the other hand, government & academic institutes segment is expected to be the most opportunistic segment during the forecast period. This can be attributed to the increased investments by government and academic institutions on the preclinical studies on the discovery and development of various drugs and medical devices. The rising outsourcing activities of such institutes is expected to drive the growth of the preclinical CRO market.
Regional Insights
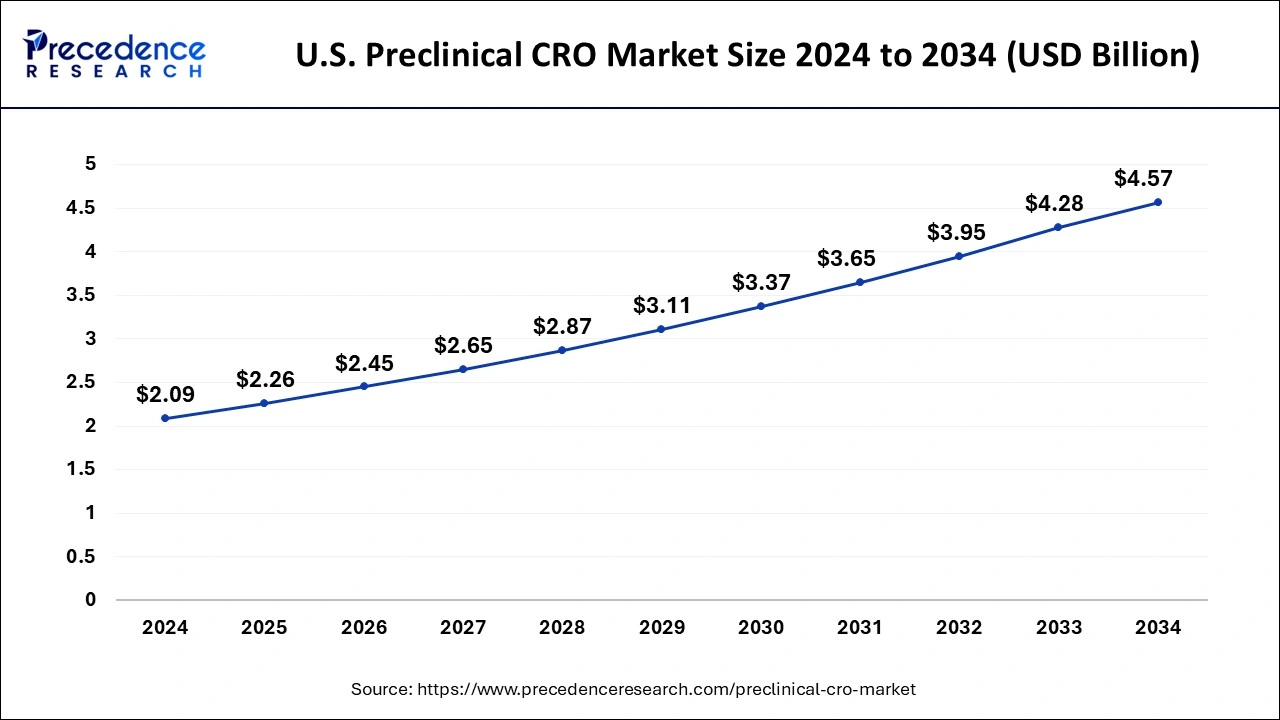
U.S. Preclinical CRO Market Size and Growth 2025 to 2034
The U.S. preclinical CRO market size is exhibited at USD 2.26 billion in 2025 and is projected to be worth around USD 4.57 billion by 2034, growing at a CAGR of 8.14% from 2025 to 2034.
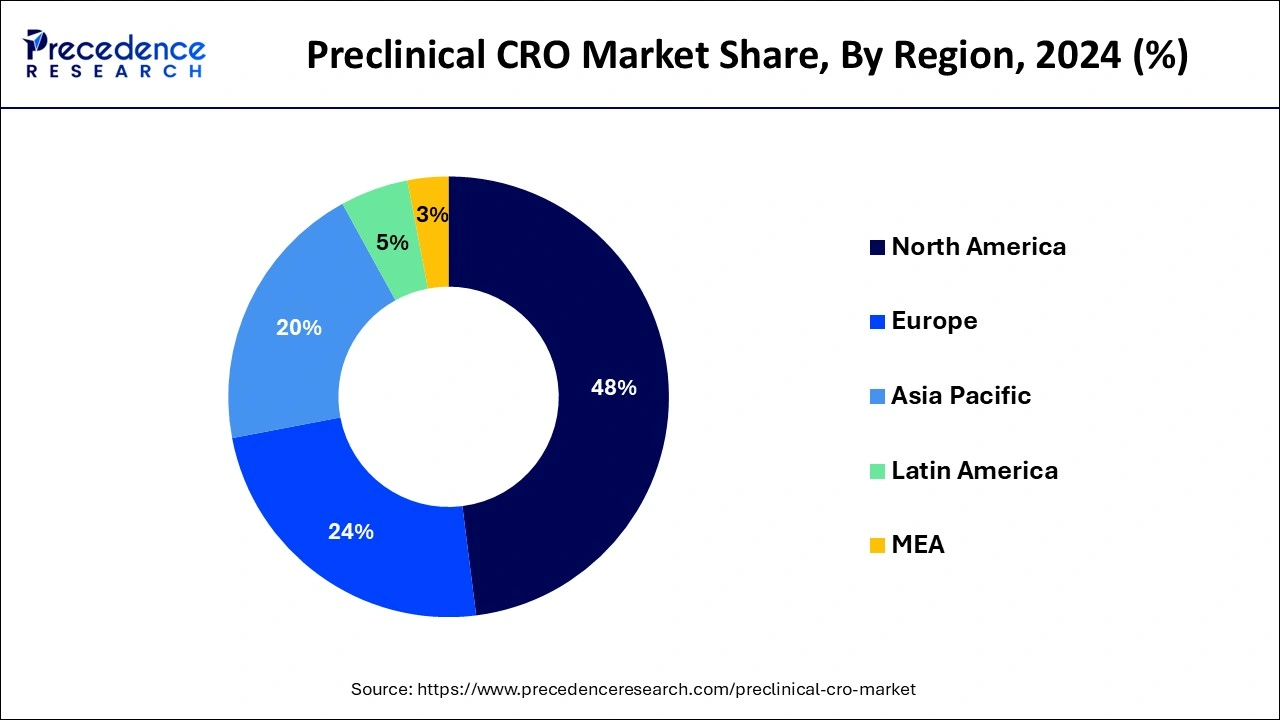
Based on region, North America dominated the global preclinical CRO market by 47.14% in 2024, in terms of revenue and is estimated to sustain its dominance during the forecast period. This can be attributed to the presence of numerous top biopharmaceutical industry players in the region. The increased volume of outsourcing activities for the preclinical phase of the drug development by the biopharmaceutical companies has fostered the growth of the market in this region. According to the Pharmaceutical Research and Manufacturers Association (PhRMA), the US conducts more than half of the research & development activities in the pharmaceutical field and also holds intellectual rights of a significant amount of new medicines. The biopharmaceutical industry accounted for around 4% of the US GDP in 2015. Therefore, the rapidly growing biopharmaceutical industry is expected to play a crucial role in the growth of the global preclinical CRO market.
Asia Pacific is estimated to be the most opportunistic market during the forecast period. This is due to the rising prevalence of chronic diseases among the population. Further, the countries like China, India, and South Korea are characterized by the presence of certain big CROs, which are known for conducting preclinical studies at low cost. Moreover, the rising number of CROs in the region is boosting the growth of the preclinical CRO market in Asia Pacific.
How is North America leading in the Preclinical CRO Market?
North America is the leading region in the preclinical CRO market with a good pharmaceutical industry and advanced research infrastructure. The region's strong emphasis on innovation, drug safety, and scientific excellence supports the market's continuous growth. Preclinical research operations become more efficient through strategic partnerships and regulatory frameworks, thus keeping North America's leadership position in this sector.
United States Preclinical CRO Market Trends:
The United States has the upper hand in the preclinical CRO market, which is largely due to the presence of numerous pharmaceutical and biotechnology companies in the country. The combination of good regulatory practices, adopting new technologies, and concentrating on the development of new drugs in their infancy stages guarantees the country will have control over outsourcing preclinical research services for a long time.
How is Asia-Pacific performing in the Preclinical CRO Market?
Asia-Pacific is the most opportunistic region because of the cost factor, skilled research professionals, and R&D capabilities being developed. The region is rapidly modernizing its healthcare system, and government initiatives are behind the foreign collaborations and investments. China, India, and South Korea are among the countries that are getting recognized for their scientific expertise and good quality preclinical research services.
China Preclinical CRO Market Trends:
China has positioned itself as a noteworthy player in the preclinical outsourcing area, mainly because of its price advantage and the growing scientific expertise. The increasing Chinese investments in biotechnology and R&D centers are also pulling global partnerships towards them, thus augmenting their power in the world preclinical CRO market.
What are the driving factors of the Preclinical CRO Market in Europe?
Europe is still the bright star in the market with its well-established base of CROs and a mature pharmaceutical ecosystem. The region is characterized by ethical and quality standards that support research driven by innovation. Germany and the UK are key players in developing new testing models that help to advance the preclinical research methodologies progressively.
Germany Preclinical CRO Market Trends:
Germany is a leading European center for preclinical research, owing to its high-tech laboratory facilities and its precision targeting studies with precision. The area's insistence on compliance, quality, and innovation creates a favorable environment for cooperation between academic research institutes and the CROs, thus fortifying the nation's market share.
How is North America leading in the Preclinical CRO Market?
North America is the leading region in the preclinical CRO market with a good pharmaceutical industry and advanced research infrastructure. The region's strong emphasis on innovation, drug safety, and scientific excellence supports the market's continuous growth. Preclinical research operations become more efficient through strategic partnerships and regulatory frameworks, thus keeping North America's leadership position in this sector.
United States Preclinical CRO Market Trends:
The United States has the upper hand in the preclinical CRO market, which is largely due to the presence of numerous pharmaceutical and biotechnology companies in the country. The combination of good regulatory practices, adopting new technologies, and concentrating on the development of new drugs in their infancy stages guarantees the country will have control over outsourcing preclinical research services for a long time.
How is Asia-Pacific performing in the Preclinical CRO Market?
Asia-Pacific is the most opportunistic region because of the cost factor, skilled research professionals, and R&D capabilities being developed. The region is rapidly modernizing its healthcare system, and government initiatives are behind the foreign collaborations and investments. China, India, and South Korea are among the countries that are getting recognized for their scientific expertise and good quality preclinical research services.
China Preclinical CRO Market Trends:
China has positioned itself as a noteworthy player in the preclinical outsourcing area, mainly because of its price advantage and the growing scientific expertise. The increasing Chinese investments in biotechnology and R&D centers are also pulling global partnerships towards them, thus augmenting their power in the world preclinical CRO market.
What are the driving factors of the Preclinical CRO Market in Europe?
Europe is still the bright star in the market with its well-established base of CROs and a mature pharmaceutical ecosystem. The region is characterized by ethical and quality standards that support research driven by innovation. Germany and the UK are key players in developing new testing models that help to advance the preclinical research methodologies progressively.
Germany Preclinical CRO Market Trends:
Germany is a leading European center for preclinical research, owing to its high-tech laboratory facilities and its precision targeting studies with precision. The area's insistence on compliance, quality, and innovation creates a favorable environment for cooperation between academic research institutes and the CROs, thus fortifying the nation's market share.
Key Companies & Market Share Insights
The market is moderately fragmented with the presence of several local companies. These market players are striving to gain higher market share by adopting strategies, such as investments, partnerships, and acquisitions & mergers. Companies are also spending on the development of improved products. Moreover, they are also focusing on maintaining competitive pricing.
In September 2020, Innoplexus and Paraxel collaborated to launch Clearinghouse, an online resource for the researchers and clinical trial sponsors that provided all the datasets and information regarding the COVID-19 virus.
The various developmental strategies like partnerships, collaborations, and joint ventures fosters market growth and offers lucrative growth opportunities to the market players.
Preclinical CRO MarketCompanies
- Wuxi AppTec
- Pharmaceutical Product Development
- Medpace, Inc.
- Charles River Laboratories International, Inc.
- PRA Health Science, Inc.
- PAREXEL
- Envigo
- Eurofins Scientific.
- Laboratory Corporation of America
- ICON Plc
Recent Developments
- In September 2025, CPDC announced the launch of Cadena Research, a preclinical CRO that provides specialized support for early-stage drug development in radiopharmaceuticals.
https://www.prnewswire.com - In April 2025, Debiopharm and Oncodesign Services signed a license agreement for the use of AbYlink™ technology, enhancing preclinical services in drug discovery for cancer and infectious diseases.
https://www.businesswire.com
Value Chain Analysis:
- R&D: Conducting scientific studies for potential drug discovery and innovating the preclinical testing methods.
Key Players: Charles River Laboratories, Labcorp Drug Development, WuXi AppTec - Clinical Trials and Regulatory Approvals: Human safety and regulatory approval are determined by clinical CROs working in tandem.
Key Players: ICON plc, Syneos Health, Labcorp Drug Development - Formulation and Final Dosage Preparation: Pharmaceutical form formulation through the combination of active and inactive ingredients with high stability and deliverability.
Key Players: Thermo Fisher Scientific (PPD), WuXi AppTec, Catalent - Packaging and Serialization: Marking and assigning identifiers for tracking, thus ensuring the quality control and authenticity of the drug products.
Key Players: Thermo Fisher Scientific (PPD), Lonza, PCI Pharma Services - Distribution to Hospitals, Pharmacies: The supply chain network is managed in such a way that the drug keeps its integrity throughout.
Key Players: AmerisourceBergen, Cardinal Health, McKesson - Patient Support and Services of Preclinical CRO: It is limited to laboratory research with no direct patient interaction, thus focusing on pre-human drug testing.
Key Players: IQVIA, Fortrea (formerly part of Labcorp), Syneos Health
Segments Covered in the Report
By Service
- Toxicology Testing
- Bioanalysis & DMPK Studies
- Chemistry
- Compound Management
- Safety Pharmacology
- Others
By End Use
- Medical Device Companies
- Biopharmaceutical
- Government & Academic Institutes
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content