Celiac Disease Treatment Market Revenue to Attain USD 1,948.18 Mn by 2033
Celiac Disease Treatment Market Revenue and Trends 2025 to 2033
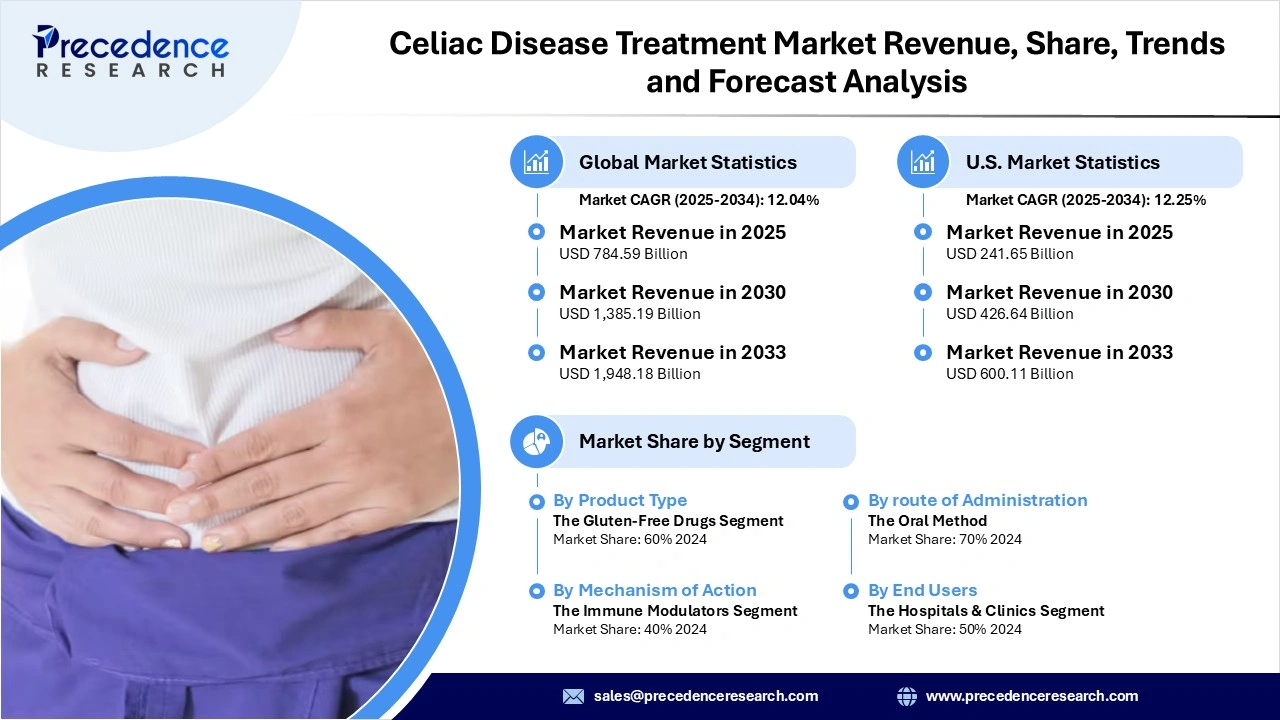
The global celiac disease treatment market revenue is valued at USD 784.59 million in 2025 and is expected to attain around USD 1,948.18 million by 2033, growing at a CAGR of 12.04% during forecast period. The market is rising because the increasing prevalence of celiac disease, greater diagnostic uptake, and therapeutic innovation are combining to expand the treated patient population and drive demand for advanced treatment solutions.
Key drivers enabling the growth of the celiac disease treatment market
Multiple fundamental product drivers are propelling growth in the celiac disease product segment. The increasing awareness of celiac disease is occurring worldwide, which has led to higher rates of diagnosis and thus a higher addressable patient population. Along with these advances in diagnostic technologies and screening protocols, they are facilitating earlier detection and treatment initiation.
The explosion of gluten-free food products and accompanying strategies for dietary management is raising awareness within the larger treatment ecosystem, thereby increasing expectations for adjunct pharmacologic treatments beyond a strict diet alone. Investments by pharmaceutical and biotech companies in innovative therapies, immune modulators, enzyme therapies, and even vaccine-related treatments are accelerating and providing hope through new mechanisms for unmet therapeutic needs. Finally, enhancements in healthcare infrastructure in some regions, specifically North America and Asia-Pacific, are facilitating more rapid adoption of treatment options and distribution of new therapies.
Segment Insights
- By Product Type, the gluten-free drugs segment dominated the market in 2024, because of the large number of patients who have been diagnosed and are utilizing gluten-free therapies for their ability to offer symptom relief and to prevent damage to the intestinal tissue from exposure to gluten.
- By Mechanism of Action, the immune modulators segment is the dominating segment of the market due to its unique method of therapy that works to directly impact abnormal immune systems that contribute to the disease course, enabling better and longer-term management of the disease in patients with celiac disease.
- By Route of Administration, the oral route segment dominated the market due to the nature of the route has been shown to result in higher patient compliance, thus is aided by active and growing developments of gluten-neutralizing drugs and immunotherapy drugs for celiac disease that can be administered orally.
- By End-Users, the hospitals and clinics segment dominates the market because of sophisticated diagnostic tools for celiac disease, inpatient gastroenterology units and access to comprehensive care for celiac disease.
- By Distribution Channel, the hospital pharmacies segment dominates the market due to an increase in prescriptions for celiac disease therapies that are being supplied by hospital or non-hospital specialty pharmacy outlets.
Regional Insights
North America leads the celiac disease treatment market due to high-quality healthcare infrastructure, high patient awareness, and significant R&D investments in countries such as the United States.
Asia-Pacific is the fastest-growing region, driven by increased access to healthcare, higher rates of diagnosis, and the middle class's greater financial capacity to alter their diets in countries like China and India to meet the growing demand for celiac disease therapies.
Celiac Disease Treatment Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 784.59 Million |
| Market Revenue by 2033 | USD 1,948.18 Million |
| CAGR from 2025 to 2033 | 12.04% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Recent Developments
- On May 27, 2025, Teva Pharmaceutical Industries, Ltd. announced that its investigational anti-IL-15 antibody candidate TEV-53408 received Fast Track designation from the U.S. Food & Drug Administration for the treatment of celiac disease. (Source: https://ir.tevapharm.com)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/7080
You can place an order or ask any questions, please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344