Circulating Tumor DNA (ctDNA) Market Revenue to Attain USD 24.09 Bn by 2033
Circulating Tumor DNA (ctDNA) Market Revenue and Trends 2025 to 2033
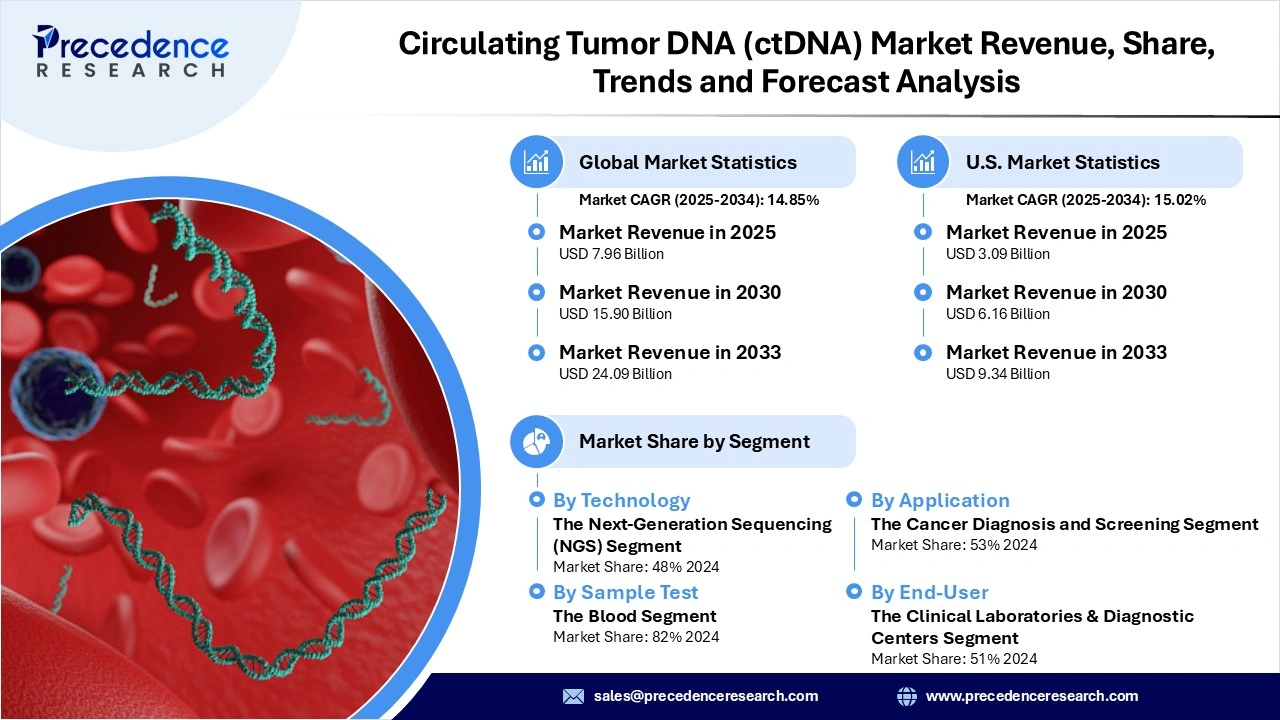
The global circulating tumor DNA (ctDNA) market revenue reached USD 7.96 billion in 2025 and is predicted to attain around USD 24.09 billion by 2033 with a CAGR of 14.85%. The market is rising primarily due to surging demand for minimally invasive cancer diagnostics, rising cancer incidence worldwide, growing adoption of precision medicine, and continuous breakthroughs in ultra-sensitive sequencing and bioinformatics technologies.
What are the key factors enabling the growth of this market?
The circulating tumor DNA (ctDNA) market's expansion is driven by a confluence of factors. Initially, the increasing global prevalence of cancer demands timely detection and monitoring, making the non-invasive nature of ctDNA testing highly appealing. Secondly, the shift towards precision oncology, where ongoing molecular insights (like ctDNA) enable improved targeted therapies and treatment adjustments, is accelerating. Third, ongoing innovations, particularly in next-generation sequencing, error mitigation, methylation assays, and fragmentomics, are enhancing detection sensitivity and specificity to unprecedented levels, even for minimally residual disease (MRD) and early assessment of treatment response, even down to single-digit percentages of relapsed tumor fractions.
Fourth, regulatory and reimbursement advancements, alongside infrastructure investments, are fostering adoption across educational, clinical, and institutional settings. Lastly, the move from tissue biopsies to "liquid biopsies" is being adopted because ctDNA biopsies more accurately reflect tumor heterogeneity, while also allowing for risk stratification and longitudinal monitoring.
Segment Outlook:
- By technology, the next-generation sequencing (NGS) segment dominated the market in 2024, owing to its multiplexing capability, deep read coverage, and ability to profile multiple genomic alterations. NGS also improves variant sensitivity and reduces the cost per variant, making it the most desirable technology for ctDNA analysis.
- By application, the cancer diagnosis and screening segment led the market in 2024, due to the growing use of ctDNA for noninvasive early detection and routine surveillance across multiple cancer types.
- By sample type, the blood segment dominated the market in 2024, because of its status as the most clinically accepted, accessible, and validated medium for ctDNA collection and analysis.
- By end-user, the clinical laboratories & diagnostic centers segment held the largest share in 2024, owing to their established infrastructure, high-throughput capacity, and regulatory accreditation to perform ctDNA assays at scale within diagnostic workflows.
Regional Insights:
North America registered dominance in the circulating tumor DNA (ctDNA) market by holding the largest share in 2024. This is mainly due to its advanced healthcare systems, substantial R&D investments, early adoption of precision medicine and research outcomes, and supportive reimbursement policies. The U.S., in particular, generates the majority of the ctDNA market revenue, supported by the presence of numerous leading diagnostics companies and a high volume of clinical trials utilizing ctDNA.
Asia Pacific is expected to witness the rapid growth in the coming years. This growth is fueled by a rising cancer incidence, increased healthcare expenditure, advancements in the molecular diagnostics sector, and growing awareness among both physicians and patients. China, India, Japan, and Southeast Asia are key contributors. Furthermore, substantial investments in genomic and cancer screening initiatives across numerous Asia-Pacific nations are poised to boost the demand for ctDNA testing.
Circulating Tumor DNA (ctDNA) Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 7.96 Billion |
| Market Revenue by 2033 | USD 24.09 Billion |
| CAGR from 2025 to 2033 | 14.85% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Recent Development:
- In September 2025, Personalis, Inc. announced a collaboration with Yale Cancer Center on a Phase II clinical trial titled CATE, evaluating ctDNA-guided adjuvant therapy with elacestrant in HR-positive, HER2-negative breast cancer patients at risk of late recurrence.(Source: https://investors.personalis.com)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/7038
You can place an order or ask any questions, please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344