Prostate Cancer Diagnostics Companies | Forecast by 2033
Prostate Cancer Diagnostics Market Top Companies, and Trends Analysis to 2034
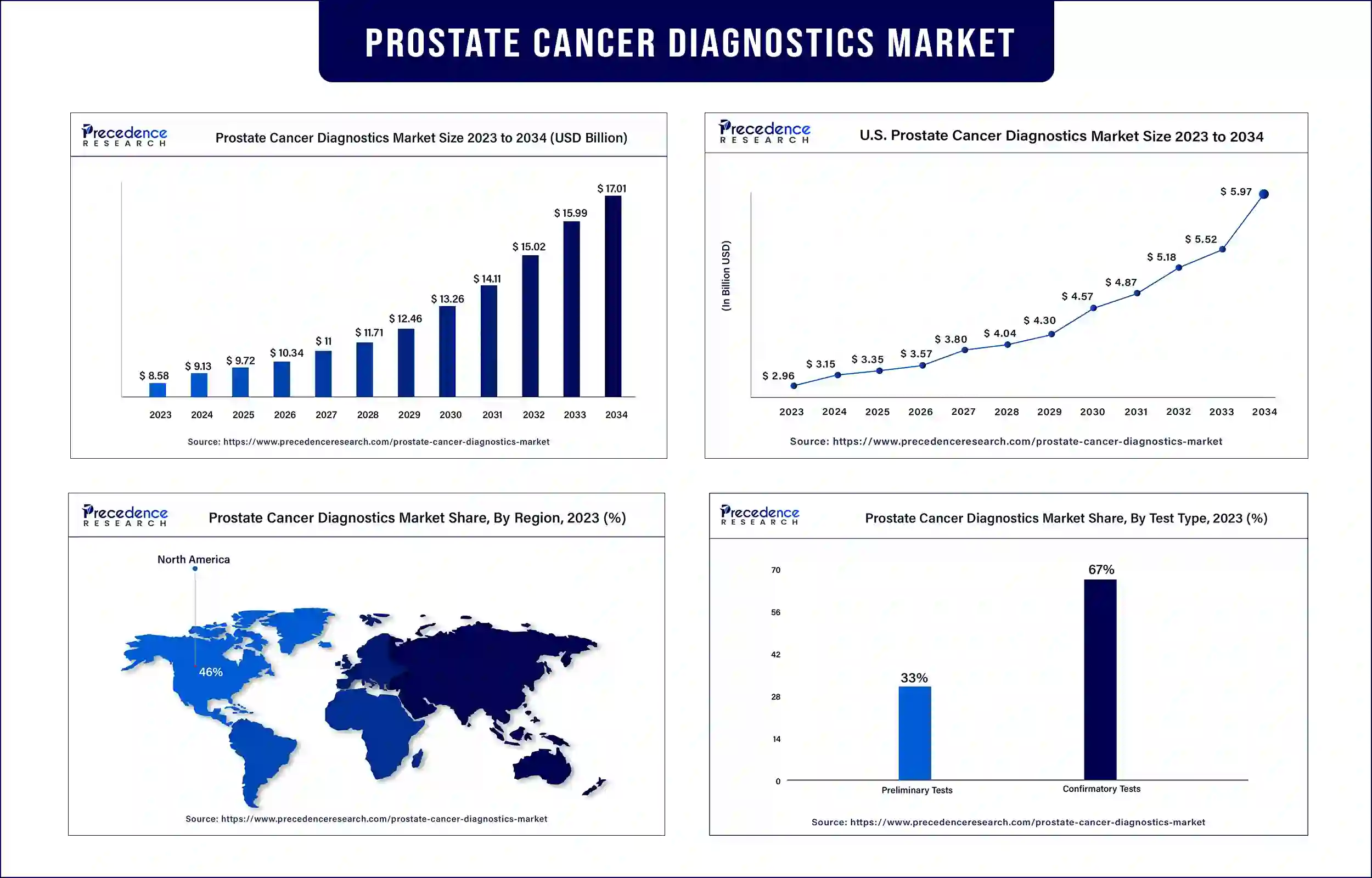
The global prostate cancer diagnostics market surpassed USD 8.58 billion in 2023 and is expected to be worth around USD 15.99 billion by 2033, growing at a CAGR of 6.42% during the forecast period. Rising cases of prostate cancer are the major reason behind the growth of the prostate cancer diagnostics market.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/4778
Market Overview
Cancer has always been one of the leading reasons of mortality across the globe. With increasing cases of cancer, prostate cancer has become a prevalent form of cancer among men, which affects their prostate glands. The prostate gland is a type of gland that makes semen fluid and is only found in males. Sometimes, with increasing age, the cells of these prostate glands start multiplying abnormally and lead to prostate cancer.
The rising incidents of prostate cancer have driven the increasing demand for diagnostics products. Prostate cancer diagnostics deals with the detection of cancer at an early stage to enhance survival rates in the future. Thus, healthcare experts are paying a significant emphasis on developing diagnosis solutions.
Prostate Cancer Diagnostics Market Emerging Trends
- Innovative technologies: Advancement in diagnostic devices leads to the growth of this market as it helps to get precise results and adoption for better treatment.
- Enhanced Medication: Due to improved drug availability, the chances of successful treatment have increased, which influences people for early detection of prostate cancer.
- Cost-effectiveness: The major reason driving the market is cost-effective diagnostics of prostate cancer which influences several people of different categories.
The growing prevalence of prostate cancer-driving the prostate cancer diagnostics market
With the changing lifestyle and environmental influences, the cases of prostate cancer are increasing. This alarming situation has become a leading threat to mortality among the male population, especially older men. The steady increase in cancer cases has driven the prostate cancer diagnostics market. With the growing importance of early detection, individuals are now more aware and seek early detection tests for prostate cancer. Since the focus of people has shifted towards early detection tests, the demand for prostate cancer market diagnostics is skyrocketing. Since increasing age is directly linked with higher chances of prostate cancer, the majority of prostate cancer patients are older men.
The rise of the elderly population increases the cases of prostate cancer across the globe. They often undergo diagnostic tests and screening to detect the symptoms of prostate cancer at an early stage. All these contribute to the escalating demand for prostate cancer diagnostics market.
The rising cases of prostate cancer are driving the cancer diagnostics market. Healthcare institutions are putting an effort into developing diagnosis solutions. Technical improvements have also contributed to the rapid development of detection tests like liquid biopsy tests, biomarker tests, PET scans, etc. All these developments are assisting the market to gain momentum.
- For instance, according to the National Institutes of Health, prostate cancer is considered the most common cancer among men and also one of the second major reasons behind the death of cancer patients after lung cancer. The occurrence and frequency of prostate cancer fluctuate in different regions of the world, which is found to be highest in North America and the lowest in South Asia.
The challenge fabricates in the circumstance that a few of the biomarkers used in prostate cancer diagnostics have the chance of lacking the specificity required to precisely differentiate among benign situations. This restraint can lead to incorrect results, which can cause needless hostile events and overtreatment. Refining the particularity of biomarkers is critical for improving the accuracy of diagnostic trials and confirming more up-to-date clinical conclusion-making.
Prostate Cancer Diagnostics Market Top Companies
- Myriad Genetics, Inc.
- F. Hoffman-La Roche AG
- MDx Health
- Proteomedix
- Beckman Coulter, Inc.
- Hologic Inc.
- Pfizer Inc.
- OPKO Health, Inc.
- Metamark Genetics, Inc
- DiaSorin S.p.A
- Quest Diagnostics
- Humasis
- Best NOMOS
- Siemens Healthcare GmbH
- Genomic Health.
- Abbott Laboratories
- Bayer AG
Recent Development by Best Nomos
- In April 2024, Best NOMOS, which is a product designing and solutions company that helps medical experts treat a variety of cancers, launched a high-innovation Compact SONALIS Ultrasound System, provided that the highest resolution, proficient in using 20 diverse probes for analysis.
Recent Development by Quest Diagnostics
- In July 2023, Quest Diagnostics, which is a leading provider of diagnostics devices, collaborated with Envision Sciences Pty Ltd., which is a clinical diagnosis company, jointly launched AmeriPath with an aim for novel prostate cancer biomarker trials.
Regional Insights
Asia-Pacific is estimated to grow at the fastest rate during the forecast period. The rising demand for prostate cancer diagnostics is due to investments by government and private companies in developing countries such as China, India, Japan, and many others. Such investments help the market players with research work to introduce innovations in the existing market to ease the detection procedure and provide accuracy in reports.
Collaborations among pharmaceutical companies help to announce some beneficial strategies for cancer patients and help the market to develop rapidly. Continuous innovation in this field helps healthcare experts to understand patients’ conditions deeply and adopt suitable treatments that help them recover early. With such practices, the reliability of patients improves, which pushes the prostate cancer diagnostics market to grow more.
- For instance, the National Pharmaceutical Pricing Authority (NPPA), which comes under the Ministry of Chemicals & Fertilizers, released a list of 390 anti-cancer non-scheduled Medicines with MRP declination up to 87%. This step benefited 22 lakh cancer patients across the country and saved approximately Rs. 800 Crores of patients.
- For instance, in October 2023, Blue Earth Diagnostics Ltd. and Sinotau Pharmaceutical Group collaborated to develop the Positron Emission Tomography (PET) imaging agent flotufolastat (18F) injection, which was earlier known as 18F-rhPSMA-7.3 in prostate cancer.
North America dominated the prostate cancer diagnostics market in 2023 due to the development of market players that add innovation to the market. The continuous investment in the government, healthcare centers, and pharmaceutical companies helps the market players with research work in diagnostic procedures. Such steps are taken to slow down the death rate of cancer patients by providing facilities and funding for diagnosis and treatment. This requires a huge number of diagnosis devices for proper screening of the patients and monitoring them throughout the treatment process. The huge demand for devices ultimately results in the development of the prostate cancer diagnostics market.
- For instance, in August 2024, the Biden-Harris administration awarded approximately $9 Million to 18 HRSA for improved access to Cancer Screening and the enhancement of follow-up treatment in unreserved communities.
Market Potential & Growth Opportunity
Focus on personalized medicine expands prostate cancer diagnostics market
The growing importance of tailored medication in prostate cancer diagnostics gives a chance to modify diagnostic methods based on distinct patient physical appearance. Participating in genetic reporting, molecular examination, and patient-precise aspects permits for a more effective and targeted diagnostic approach. This modified method improves the precision of prostate cancer diagnosis as well as opens opportunities for the growth of modified treatment strategies, positioning the wider trend in health care in the direction of more modified and patient-centered attention. Constant surveys related to prostate cancer, such as circulating tumor cells, specific proteins, and genetic mutations, generate chances for the growth of highly delicate and precise diagnostic tests.
- In March 2024, GE Healthcare, which is a leading global medical technology company that provides a portfolio of products and services used for diagnosis and treatment, launched an AI-powered software to support prostate measurements and calculations and beneficial in prostate imaging and ultrasound guide.
Prostate Cancer Diagnostics Market Highlights
| Report Attribute | Key Statistics |
| Market Revenue in 2024 | USD 9.13 Billion |
| Market Revenue by 2033 | USD 15.99 Billion |
| CAGR | 6.42% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2023 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Prostate Cancer Diagnostics Market News
- In February 2024, DiaCarta Inc. and OncoAssure Ltd collaborated to launch a prostate cancer test with the aim of early detection of the disease. It is planned to detect patients with a minor risk of prostate cancer reappearance.
- In May 2024, Cortechs.ai announced the launch of OnQ Prostate, which is a novel MRI solution for prostate cancer diagnosis. This launch has helped the company to expand its market in urologic oncology.
Market Segmentation
By Test Type
- Preliminary Tests
- PSA Tests
- Free PSA Test
- Total PSA Test
- Other Preliminary Tests
- Confirmatory Tests
- Pca3 Test
- Trans-Rectal Ultrasound
- Biopsy Test
By Type
- Adenocarcinoma
- Interstitial Cell Carcinoma
- Other
By End-Use
- Hospitals
- Home Care
- Outpatient Facilities
- Research & Manufacturing
Buy this Research Report@ https://www.precedenceresearch.com/checkout/4778
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308