Renal Denervation Devices Market Revenue to Attain USD 3.91 Bn by 2033
Renal Denervation Devices Market Revenue and Trends 2025 to 2033
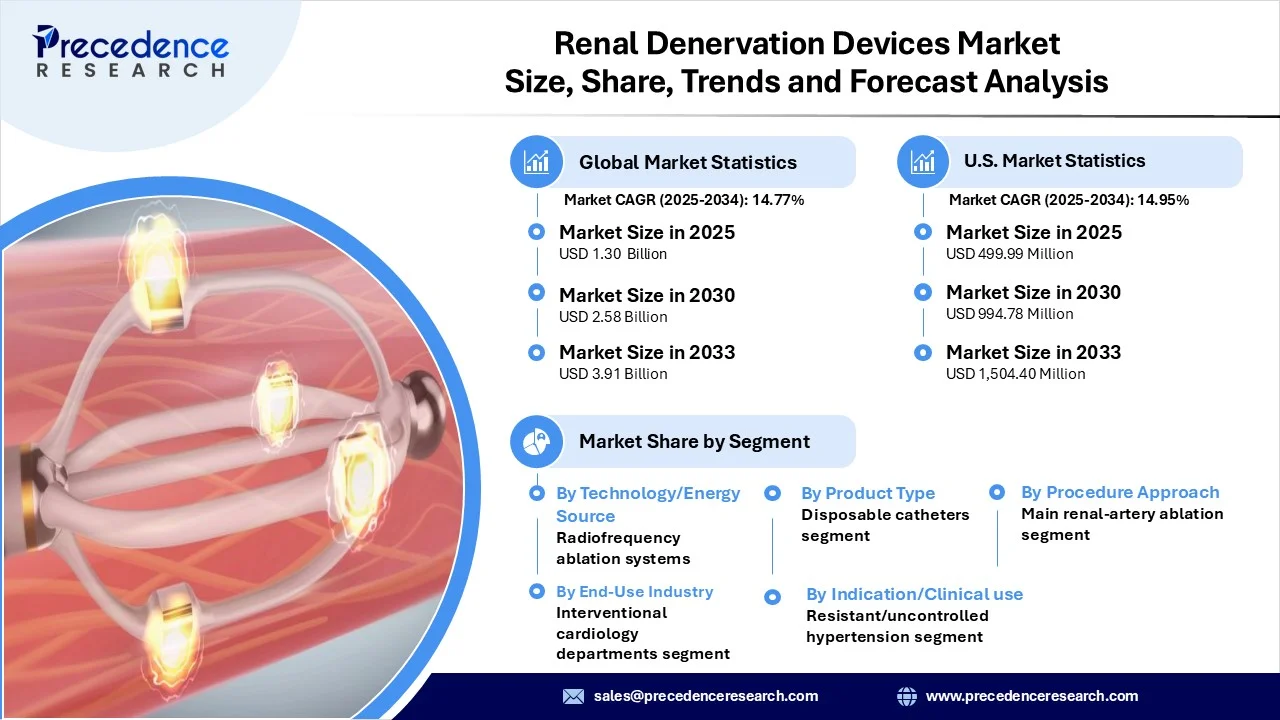
The global renal denervation devices market revenue reached USD 1.30 billion in 2025 and is predicted to attain around USD 3.91 billion by 2033 with a CAGR of 14.77%. The market is expanding at a rapid pace, driven by rising hypertension prevalence, technological advancements in medical devices, and increasing adoption of minimally invasive cardiovascular therapies worldwide.
Emerging Trends and Growth Outlook in the Renal Denervation Devices Market
The renal denervation devices market refers to the medical devices that are designed to disrupt overactive renal sympathetic nerves to treat patients with resistant hypertension through minimally invasive catheter-based procedures. This market is experiencing significant growth in the face of persistent global concerns regarding hypertension, which is driving the demand for innovative therapies beyond pharmacotherapy. Increasing awareness of renal denervation as an alternative to lifelong medication is also driving market growth. Ongoing advancements in catheter technology, regulatory support, and high adoption rates of these devices are contributing to the market's growth. Moreover, the growing demand for long-term cost-effective solutions in hypertension management creates immense opportunities.
Segmental Analysis
- By technology / energy source, the radiofrequency (RF) ablation systems segment dominated the market in 2024, owing to these systems’ proven safety, validation in multiple clinical trials, and being the first and most widely adopted renal denervation modality.
- By product type / system component, the disposable catheters segment led the market in 2024. This is mainly due to their advantages in safety, efficiency, and regulatory compliance. These single-use catheters significantly reduce the risk of cross-contamination and infection, aligning with strict hospital protocols and infection control standards.
- By procedure approach / clinical technique, the main renal-artery ablation only segment dominated the market in 2024 due to its widespread acceptance by physicians as the standard approach, established clinical efficacy, and procedural simplicity.
- By indication / clinical use, the resistant / uncontrolled hypertension remains the dominant segment in 2024, as it represents the largest pool of patients in urgent need of alternative treatment options. Renal denervation has emerged as a promising solution for these patients, offering a minimally invasive approach to reduce sympathetic nerve activity and achieve better blood pressure control.
- By end-user / buyer, the interventional cardiology departments / cardiologists segment dominated the market in 2024 due to their central role in diagnosing and managing hypertension and cardiovascular conditions. They leverage expertise via catheter-based therapies to enhance the capability of renal denervation as treatment and represent the leading user of renal denervation in the cardiovascular treatment paradigm.
- By sales channel / commercial model, the direct sales segment dominated the market as manufacturers focus on establishing strategic relationships with large hospital systems to expand efficiency of procurement, physician training, and accelerate the process of renal denervation in larger systems.
Regional Analysis
North America registered dominance in the renal denervation devices market by holding the largest share in 2024. This is due to the early approvals by regulatory bodies, interventional infrastructure that leads the world, and the adaptation of technology by clinicians. North America also has very high levels of financial support for healthcare systems in addition to established hospitals across the region that are addressing the needs of renal denervation.
Asia Pacific is emerging as the fastest-growing region due to an increase in clinical trials, regulatory approvals, and investment aimed at improving healthcare systems. Rising government support to spread awareness of hypertension management further supports market growth. With growing hospitals and the continued evolution of interventional technologies, the region is anticipated to flourish in the market.
Renal Denervation Devices Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 1.30 Billion |
| Market Revenue by 2033 | USD 3.91 Billion |
| CAGR from 2025 to 2033 | 14.77% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Development
- In June 2024, South Korean medtech firm DeepQure launched an Early Feasibility Study for its renal denervation device, HyperQure, targeting resistant hypertension. The prospective, multicenter, single-arm, open-label trial follows FDA approval under an Investigational Device Exemption (IDE) and will evaluate the device’s safety and efficacy in 15 patients across U.S. hospitals. (Source: https://www.medicaldevice-network.com)
Key Players in the Renal Denervation Devices Market
- Ablative Solutions, Inc.
- Abbott (St. Jude Medical heritage technologies)
- Avinger / related interventional companies (adjacent technologies & mapping)
- Boston Scientific Corporation (including Vessix heritage technologies)
- Kona Medical, Inc.
- Mercator MedSystems, Inc.
- Medtronic plc (Symplicity RF systems)
- Neuravi / Renal-focused interventional firms (emerging tech partners)
- PulseCath Technologies / investigational device firms
- ReCor Medical, Inc. (Paradise™ ultrasound system)
- Renal Dynamics Limited
- SoniVie / CardioSonic (historical ultrasound/early programs)
- Symple Surgical, Inc.
- Terumo Corporation
- Vascular / specialty device firms offering RDN-capable platforms (e.g., Vessix lineage via Boston Scientific)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6814
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344