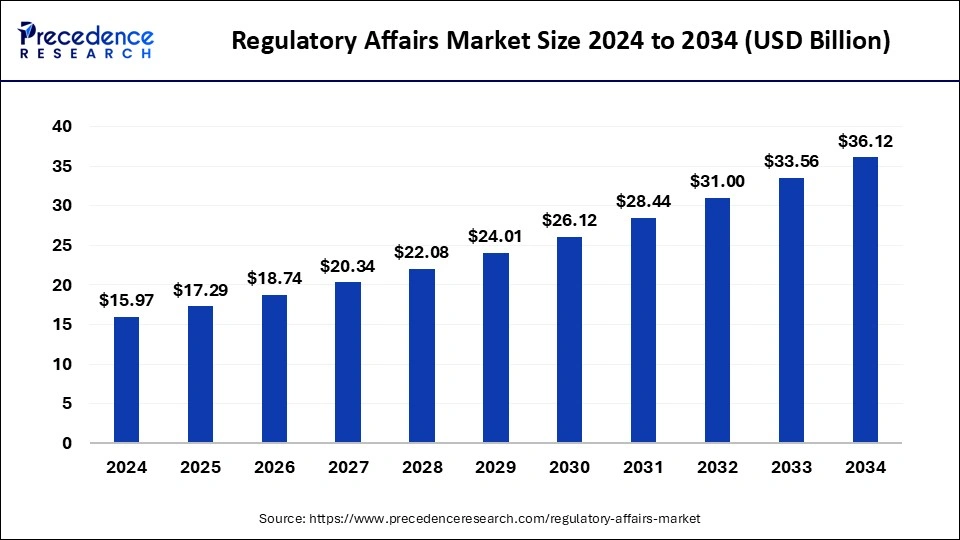
Regulatory Affairs Market Size and Forecast 2025 to 2034
The global regulatory affairs market size accounted for USD 15.97 billion in 2024 and is predicted to increase from USD 17.29 billion in 2025 to approximately USD 36.12 billion by 2034, expanding at a CAGR of 8.53% from 2025 to 2034.
Regulatory Affairs Market Key Takeaways
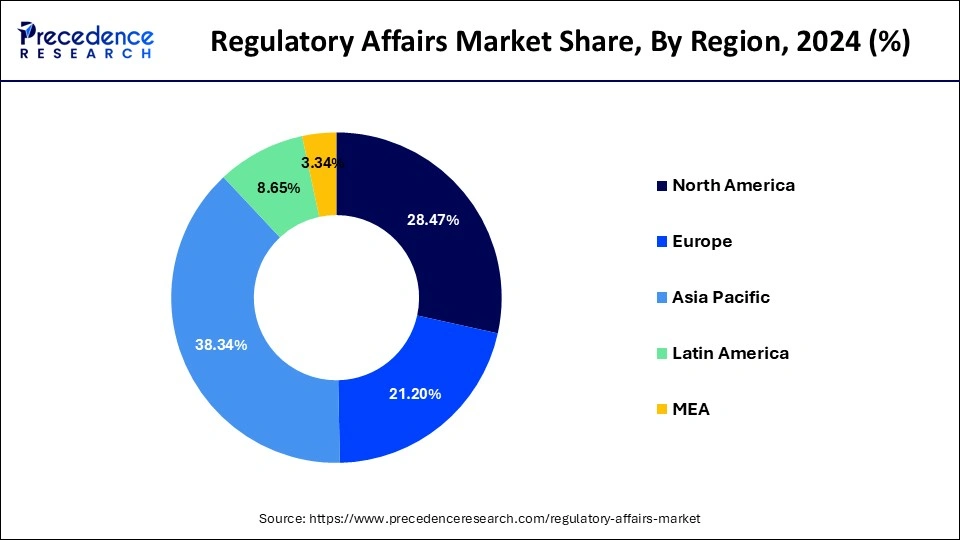
- The Asia Pacific led the global market with the largest market share of 38.34% in 2024.
- North America is expected to expand at the fastest CAGR of 8.61% between 2025 and 2034.
- By services, the regulatory writing and publishing segment has captured more than 36.5% of revenue share in 2024.
- By service, the legal representation segment is growing at a remarkable CAGR of 9.53% between 2025 and 2034.
- By category, the medical devices segment held the largest market share of 43.68% in 2024.
- By category, the drugs segment is projected to grow at a notable CAGR of 8.73% between 2025 and 2034.
- By indication, the oncology segment has contributed more than 32.07% of revenue share in 2024.
- By indication, the immunology segment is expected to grow at a CAGR of 8.91% between 2025 and 2034.
- By stage, the clinical studies segment has generated around 48.87% of revenue share in 2024.
- By stage, the PMA segment is expected to expand at the fastest CAGR of 8.52% between 2025 and 2034.
- By service provider, the outsourced segment led the global market with the biggest market share of 56.60% in 2024.
- By service provider, the In-house segment is projected to grow at a CAGR of 6.92% between 2025 and 2034.
- By company, the medium segment has captured around 46.80% of revenue share in 2024.
- By end user, the pharmaceutical companies segment has accounted around 41.52% of revenue share in 2024.
Asia Pacific Regulatory Affairs Market Size and Growth 2025 to 2034
The Asia Pacific regulatory affairs market size was exhibited at USD 6.12 billion in 2024 and is projected to be worth around USD 14.34 billion by 2034, growing at a CAGR of 8.91% from 2025 to 2034.
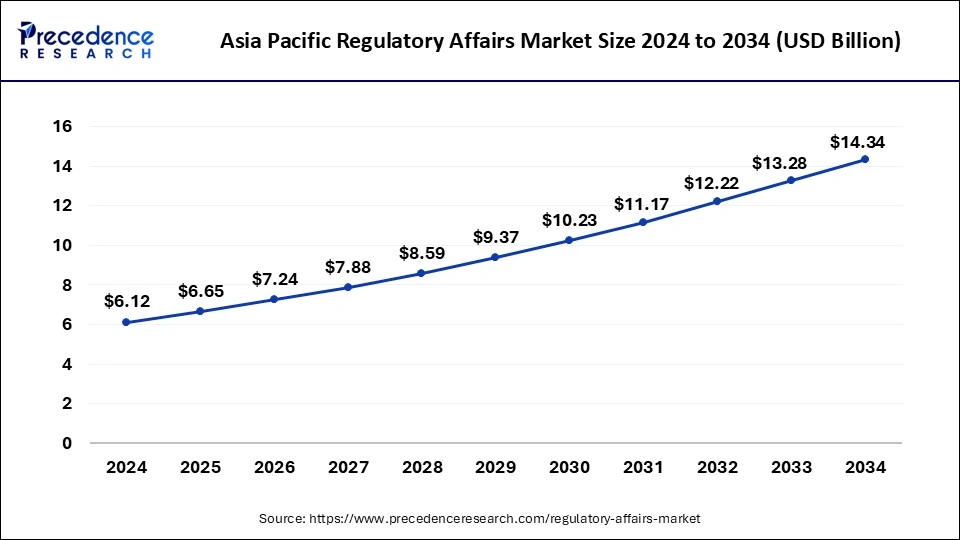
The Asia Pacific region has generated more than 38.34% of revenue share in 2024 and showed the fastest growth and hence dominated the segment for a considerable period of time and he's foreseen to continue the same during the forecast period. It is expected to expand in the future as a result of the recent regulatory advancements and rapidly increasing clinical studies and trials. A huge cost saving strategy of the region has proved to be a driving factor for the growth of the market. The huge availability of quality labor which is present in this region has boosted the market size tremendously. The rapidly increasing geriatric population proves to be another reason for the market growth.
- The China regulatory affairs market size was estimated at USD 2,126.60 million in 2024 and is anticipated to reach around USD 5,085.00 billion by 2034, growing at a CAGR of 9.13% from 2025 to 2034.
- TheIndia regulatory affairs market size reached USD 1,302.40 million in 2024 and is estimated to surpass around USD 3,050.20 billion by 2034, expanding at a notable CAGR of 8.90% from 2025 to 2034.
- TheJapan regulatory affairs market size surpassed USD 1,872.00 million in 2024 and is projected to hit around USD 4,004.20 billion by 2034, with a CAGR of 8.82% from 2025 to 2034.
Regulatory Affairs Market Growth Factors
- The growing demand for orphan drugs, personalized medicines, immune therapies, combination therapies, specialty therapies
- The requirements in the regulations and a growing number of diseases that require different types of vaccines for prevention and therapies for cure.
- The increased number of diseases also requires therapies and supportive guidelines from the government to ensure quality and safety.
- The outbreak of the pandemic.
- The rapid demand of the medicines in order to treat the infection
- Increased the demand for regulatory processes in order to create a safe and effective zone in order to conduct multiple clinical trials
A flexible nature in the introduction of COVID products has been demanded by the regulatory authorities in order to maintain the quality and effectiveness of the products. The guidelines and norms which have been launched by the regulatory authorities regarding the pandemic and use of emergency products impacted the growth of the market considerably.
The rapid advancement which has been made in the field of business, the changes observed in the field of personalized medicines, the rapid need for increased focus towards the core business strategies and the competitive nature of the key market players are the major growth factors that contribute to the development of the market during the forecast period. The lucrative offers which are provided by the key market players by outsourcing there are services and facilities have also proved to be a major driving force for the growth of the market during the forecast. The medium to large scale industries have played a major role by contributing considerably to the growth of the market.
Market Scope
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 8.53% |
| Market Size in 2025 | USD 17.29 Billion |
| Market Size by 2034 | USD 36.12 Billion |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Largest Market in 2023 | Asia Pacific |
| Fastest Growing Market | North America |
| Segments Covered | Services, Category, Indication, Stage, Service Provider, Company, End User, Geography |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Services Insights
On the basis of services, the regulatory publishing and writing sector has dominated the segment as a result of the high revenue return recorded by it. As a result of the rapid increase in the outsourcing of writing and publishing services in order to save the investment made by the company on the employees this segment is experiencing a tremendous growth. The medium and large sized industries involved in the medical device and biopharma sector majorly perform this type of business. With a view to develop the core competencies the major pharma companies are outsourcing the regulatory publishing and writing services in order to increase their space. The segment of legal representation is foreseen to record the fastest growth as a result of its increasing demand during the forecast period. As a result of the rapid increasing complexity in the field of rules and regulations regarding the health care sector and the rapidly growing reforms in the market this segment is projected to produce a high revenue return during the forecast period.
The fast-developing countries belonging to the Asia Pacific and the Middle Eastern region have projected a tremendous growth under this segment. A legal representation is required to be possessed by the companies which are based in Europe in order to conduct quality clinical trials in the region itself. The required registration is essential under the European Union which is mandatory for each and every company which has its set-up in this region. The other segments which are discussed under this heading include regulatory consulting and product registration.
Global Regulatory Affairs Market, By Service, 2022-2024 (USD Million)
| By Service | 2022 | 2023 | 2024 |
| Regulatory Consulting | 2,211.3 | 2,386 | 2,576.8 |
| Legal Representation | 2,088.4 | 2,279.7 | 2,490.6 |
| Regulatory Writing & Publishing | 5,009.5 | 5,397.5 | 5,821 |
| Product Registration & Clinical Trial Applications | 3,528.5 | 3,833.5 | 4,168.6 |
| Other Services | 812.2 | 858.8 | 908.4 |
Category Insights
The segment of medical devices has shown a tremendous result by recording the largest revenue return of around 39.5%. The segment is foreseen to project a tremendous growth and uphold its dominant status in the market throughout the future period. the increasing trend of outsourcing multiple activities regarding medical devices is mainly responsible for according the tremendous growth seen in the market. In order to increase the focus over core competencies these major market players are making use of the outsourcing facilities.
Therapeutics and diagnostics are the other categories under the medical devices which have helped show a tremendous growth during the forecast period. The regulatory rules and norms regarding the medical devices prove to be a quality filtration regarding the efficacy of the products and safety. It also increases the medical device transparency which is beneficial for the insurer and the manufacturer. The next highest growth is showcased by the segment of drugs which includes the parts of generics and innovator. This segment has also proved to be very beneficial for the growth of the market during the forecast period.
Indication Insights
On the basis of indication, the segment of oncology has presented the maximum growth as a result of the increasing cases of cancer all over the world. The increasing demand for effective therapeutic procedures and safe drugs as options is it a sponsible for the growth of the market. It also proves to be a profitable market among all the others as a result of the biotechnology and pharmaceutical companies with support it. The rapid research and development which is carried out by the key market players also helps to record a tremendous growth. The rapid modernization which has been seen all over the world has boosted the number of patients who require advanced cancer treatment.
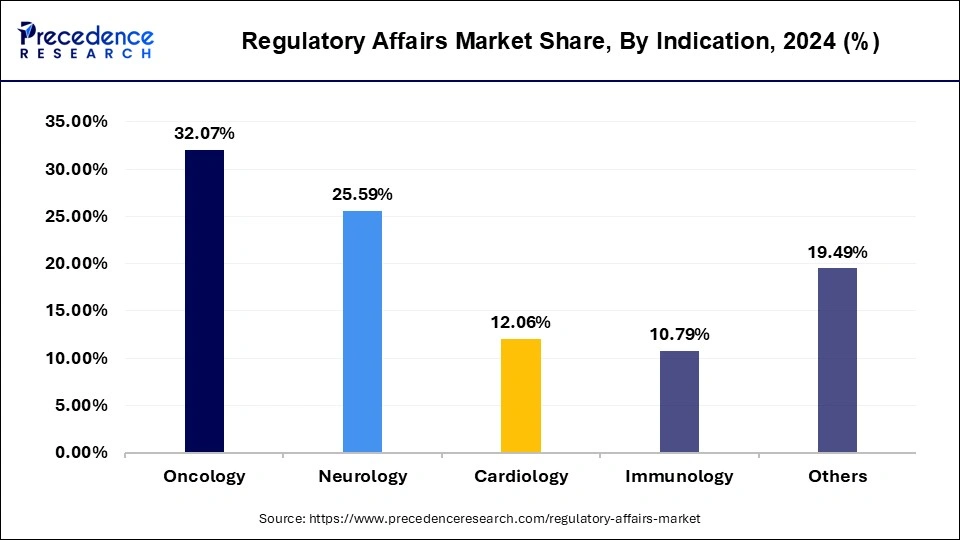
The next segment which has recorded the fastest growth according to the market is the immunology segment as a result of its contribution to the cardiovascular, inflammatory diseases and neurological disorders. As the immune cells a present in the entire body of an individual, this market has emerged as the fastest growing sector during the forecast period.
Global Regulatory Affairs Market, By Indication, 2022-2024 (USD Million)
| Indication | 2022 | 2023 | 2024 |
| Oncology | 4,375.0 | 4,730.6 | 5,119.9 |
| Neurology | 3,501.0 | 3,780.3 | 4,085.8 |
| Cardiology | 1,638.2 | 1,775.1 | 1,925.2 |
| Immunology | 1,462.2 | 1,586.2 | 1,722.3 |
| Others | 2,673.5 | 2,883.2 | 3,112.2 |
Stage Insights
On the basis of stage, the segment of clinical studies has dominated the market as a result of its largest share in the revenue system. As a result of the new diseases which are emerging in the market and the rapid rise of various chronic diseases among the people proved to be the major factors responsible for the tremendous growth of the market during the forecast period. The rapidly increasing go to search and development involves these clinical trials and studies which help the key market players to land to a conclusion in order to fulfill the health care needs of the society. In order to perform the clinical studies under strict guidance and to maintain its transparency, regulations have been laid.
The authenticity of the clinical studies is of great importance as they are further applied to the human beings for further studies. A faster growth is recorded by the preclinical segment as a result of the rapid demand of effective drugs in order to treat conditions such as the infection caused by coronavirus, ebola and the zika virus. The rapid increase in the number of cases belonging to the neurological and cardiovascular diseases has also boosted the demand for effective treatment options.
Global Regulatory Affairs Market, By Stage, 2022-2024 (USD Million)
| Stage | 2022 | 2023 | 2024 |
| Preclinical | 1,787.9 | 1,940.3 | 2,107.7 |
| Clinical studies | 6,684.21 | 7,218.4 | 7,802.4 |
| PMA | 5,177.7 | 5,596.7 | 6,055.2 |
Service Provider Insights
On the basis of service provider, the segment of outsourcing how's it dominated the market as a result of the rapid growing trend which is followed by the larger companies and manufacturers in order to avoid further investments on employees. It has recorded the maximum revenue return over the period of time which has proved to be a growing factor. this trend of outsourcing the services has been followed by many key market players belonging to the health care industry in order to decrease the total cost of production and investment made while training of the staff.
Specific technical knowledge is made use of, while outsourcing the services and facilities in order to obtain maximum output. The geographic expertise which are involved while outsourcing the services also proves to be a major growth factor for the market. A significant growth has been shown by the in-house segment under service providers and proves to be the second fastest growing market during the forecast period.
Global Regulatory Affairs Market, By Service Provider, 2022-2024 (USD Million)
| By Service Provider | 2022 | 2023 | 2024 |
| In-house | 6,087.8 | 6,492.4 | 6,929 |
| Outsource | 7,562 | 8,263 | 9,036.4 |
Company Insights
On the basis of company, the market has been dominated by the companies belonging to the medium size as a result so that's of its largest share as compared to the total revenue produced by the market. The established providers belonging to the miss medium sized sector which are particularly the private ones is foreseen to make a major contribution to the growth of the market during the forecast period.
The company is belonging to the medium sector have their presence in selected or multiple markets all over the globe which proves to be very beneficial for its future growth. The leading pharmaceutical companies, medical device industries and biotechnology industry belong to the large-scale sector which provide a huge range to the service providers.
Global Regulatory Affairs Market, By Company, 2022-2024 (USD Million)
| By Company | 2022 | 2023 | 2024 |
| Small | 3,651.3 | 3,924.9 | 4,222.8 |
| Medium | 6,388.1 | 6,908.5 | 7,478.2 |
| Large | 3,610.4 | 3,922 | 4,264.4 |
End User Insights
On the basis of end user, the pharmaceutical sector has dominated the segment as a result of its largest share in the market going to its fastest growth. the main reason for the growth of the market is the rapid increase in demand of advanced pharmaceutical products. The commercialization and marketing of the newly launched drugs approved by the FDA has proved to be a driving factor for the growth of the market. The second largest segment under this heading is of the biotechnology companies which has shown a tremendous growth during the forecast period.
Recent Developments
- In 2019 - Syntax and Accel landed into a collaboration in order to widen the client base in the region of Europe. The multiple strategies like mergers, collaborations, acquisitions help the market to record rapid growth and introduce lucrative offers to the major players.
Regulatory Affairs Market Companies
- Accell Clinical Research, LLC
- Genpact
- Promedica International
- WuXi AppTec, Inc.
- Criterium, Inc.
- PRA Health Sciences
- Medpace
- Charles River Laboratories International
- PAREXEL International Corp., Inc.
- Freyr
- ICON plc
- Covance, Inc.
Segments covered in the report
By Services
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
- Writing
- Publishing
- Product Registration & Clinical Trial Applications
- Other Services
By Category
- Drugs
- Innovator
- Preclinical
- Clinical
- Post Market
- Generics
- Preclinical
- Clinical
- Post Market
- Innovator
- Biologics
- Biotech
- Preclinical
- Clinical
- Post Market
- ATMP
- Preclinical
- Clinical
- Post Market
- Biosimilars
- Preclinical
- Clinical
- Post Market
- Biotech
- Medical Devices
- Diagnostics
- Preclinical
- Clinical
- Post Market
- Therapeutics
- Preclinical
- Clinical
- Post Market
- Diagnostics
By Indication
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
By Stage
- Preclinical
- Clinical studies
- PMA
By Service Provider
- In-house
- Outsourced
By Company
- Small
- Medium
- Large
By End User
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of the Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting