What is the Viral Clearance Market Size?
The global viral clearance market size was calculated at USD 1.13 billion in 2025 and is predicted to increase from USD 1.38 billion in 2026 to approximately USD 7.86 billion by 2035, expanding at a CAGR of 21.40% from 2026 to 2035. The viral clearance market is driven by the growing demand for biopharmaceutical products.
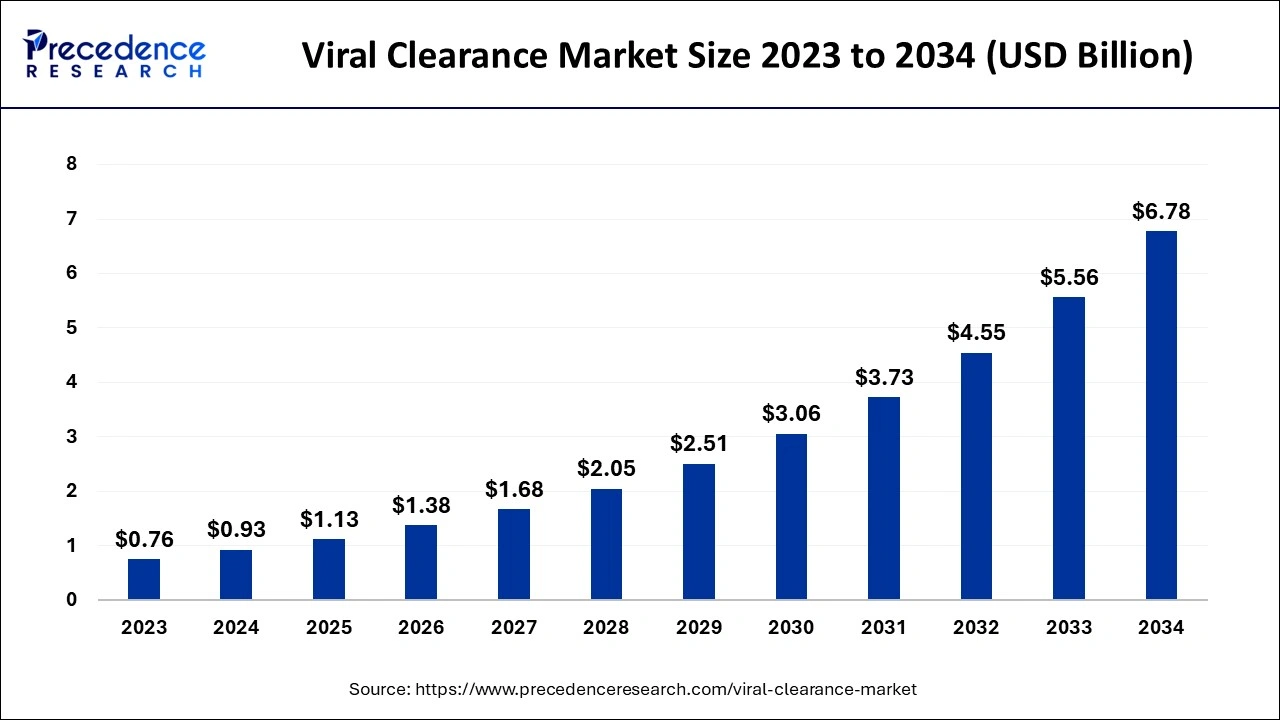
Viral Clearance Market Key Takeaways
- The global viral clearance market was valued at USD 0.93 billion in 2025.
- It is projected to reach USD 6.78 billion by 2035.
- The viral clearance market is expected to grow at a CAGR of 21.98% from 2026 to 2035.
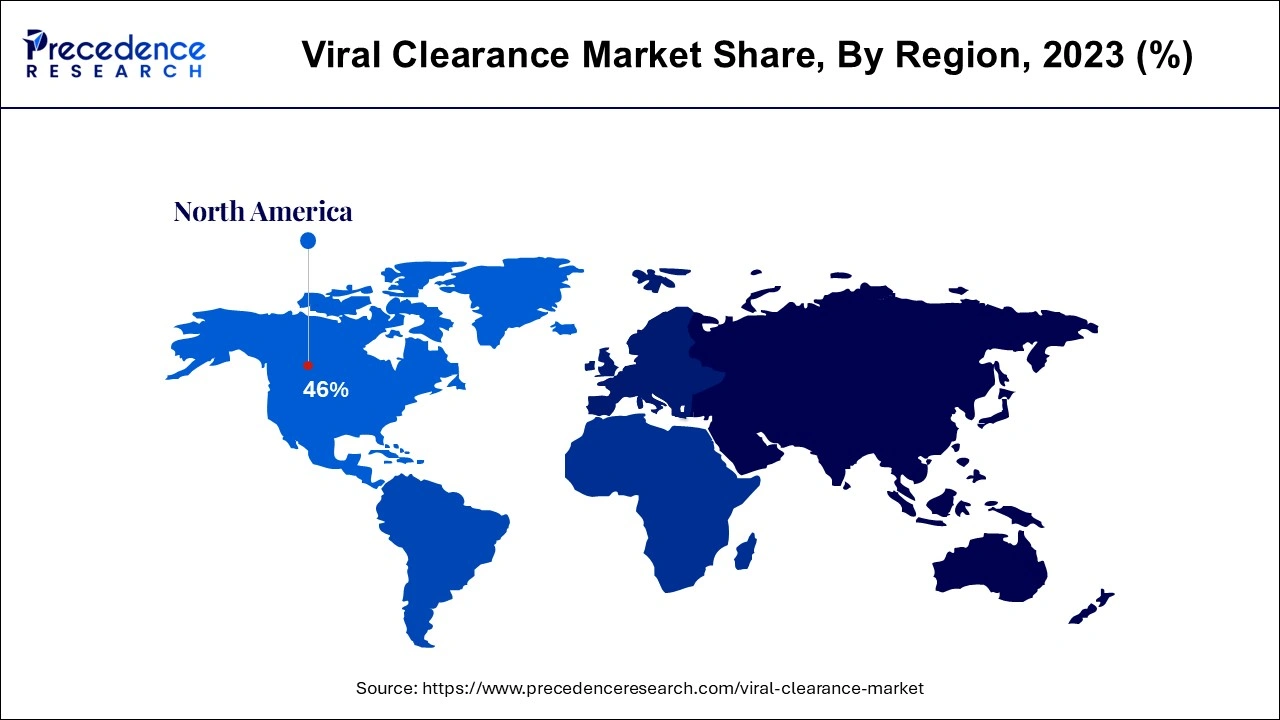
- North America dominated the global viral clearance market with the largest market share of 46% in 2025.
- Asia Pacific is expected to witness the fastest growth during the forecast period.
- By method, the viral removal segment dominated the market in 2025.
- By application, the recombinant proteins segment led the market in 2025.
- By end-user, the pharmaceutical and biotechnology companies segment dominated the viral clearance market in 2025.
- By end-user, the contract research organizations segment is expected to grow at a significant rate in the market during the forecast period.
Market Overview
Viral clearance is essential for developing biologics and is necessary to ensure product safety. One way to control product contamination is to ensure the production process can sufficiently remove any potential viral contaminants. In order to manufacture human monoclonal antibodies, specialized unit operations such as low pH inactivation, viral filtering, and chromatographic separation are used to remove the virus. The rapid expansion of cells and gene therapy, which frequently entails modifying viruses or viral vectors to treat genetic illnesses or cancer, makes viral clearance even more crucial. To guarantee that no dangerous viral impurities are left in the finished product, these treatments must pass stringent viral safety testing. International standards established by health agencies such as the WHO need to be followed in viral clearance procedures. No matter where biopharmaceutical goods are produced or disseminated, these criteria guarantee they are viral contamination-free.
How is AI Helping the Viral Clearance Market to Grow?
Developing each viral clearance step for creating a novel antibody in wet laboratory tests for process characterization research requires time, money, and effort. However, machine learning techniques may facilitate creating and improving viral clearance unit operations for novel therapeutic antibodies. They can predict the viral clearance performance, significantly accelerating the viral clearance validation process.
Viral Clearance Market Growth Factors
- Viral clearance services are in high demand as a means of guaranteeing the safety and effectiveness of pharmaceutical products due to the rising incidence of chronic illnesses and the creation of novel biologics.
- Advancements in technology, such as creating more effective filtering and inactivation techniques, are increasing the efficiency and economy of viral clearance procedures.
- Pharmaceutical and biotechnology businesses are increasingly outsourcing viral clearance activities from contract research organizations (CROs) to focus on their core expertise and cut operating expenses.
Viral Clearance Market Outlook
- Industry Growth Overview: The viral clearance market is steadily increasing as the production of biologics is on the rise, regulatory demands have increased, and the issue of product safety in biopharmaceutical manufacturing is gaining more attention. The market is also gaining use with further improvement in filtration technology and validation services.
- Major Investors: Through their viral clearance technologies and services, large companies within the life-science industry, such as Thermo Fisher Scientific, Sartorius, and Merck KGaA, have invested heavily in viral clearance technologies and services. These facilities continue to invest much capital in research and development, capacity, and strategic acquisitions.
- Startup Ecosystem: New entrants include Texcell, Vironova, and Clean Cells that are developing special viral safety tests, validation, and sensitive detection systems.
Market Scope
| Report Coverage | Details |
| Market Size by 2035 | USD 7.86 Billion |
| Market Size in 2025 | USD 1.13 Billion |
| Market Size in 2026 | USD 1.38 Billion |
| Market Growth Rate from 2026 to 2035 | CAGR of 21.40% |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Product Type, End use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Driver
Rising demand for biologics
Since biologics contain molecules such as nucleic acids and proteins, they are vulnerable to fungi, bacteria, and viruses. Thus, specific viral clearance techniques are needed to reduce any possible viral contamination. Viral contamination in biologics emphasizes the importance of reliable viral clearance procedures in previous records. Businesses are incentivized to allocate resources toward these procedures to avoid product recalls and guarantee patient safety.
Restraint
High costs of viral clearance services
Viral clearance necessitates advanced laboratory equipment and highly qualified workers. Operating and maintaining this equipment adds to overall service costs, which include operational, maintenance, and calibration charges. High service prices may stifle innovation in biopharmaceutical businesses, limiting the amount of cash available for R&D in other crucial areas.
Opportunity
Advancements in viral clearance technologies
Filtering techniques like ultrafiltration and nanofiltration increase viral elimination effectiveness. These technologies improve the yield of the product by filtering out a wider variety of viral sizes. Technology suppliers can set themselves apart in the market by offering customized viral clearance solutions for particular goods or procedures. Creating industry consortia to tackle shared viral clearance difficulties might result in pooled resources, expertise, and technological advancements, thus propelling the market's growth.
Segment Insights
Method Insights
The viral removal segment dominated the viral clearance market in 2024. This is due to the rising importance of viral removals in biologics production. The need for viral clearance technologies has been driven by the rising demand for biological medications, including gene therapies, vaccines, and monoclonal antibodies. Since biologics are produced from living things and are susceptible to viral contamination, eliminating viruses from their source is a crucial step in the manufacturing process. As the development of biologics continue to expand, the necessity for effective viral elimination methods increases. Viral safety is receiving more attention as public and regulatory worries about viral contamination rise, particularly during pandemics like COVID-19.
Application Insights
The recombinant proteins segment led the viral clearance market in 2024 due to the increasing production of recombinant proteins. The rising creation of recombinant proteins is supported by new technologies in developing viral clearance methods, such as robust inactivation methods, high-performance liquid chromatography (HPLC), and viral filtering. To demonstrate their ability to eliminate any possible viral pollutants, every step in the viral clearance process must be confirmed, including virus inactivation and removal.
End-user Insights
The pharmaceutical and biotechnology companies segment dominated the viral clearance market in 2024. These companies heavily use viral clearance during the manufacturing of pharmaceutical products. There is a high possibility of viral contamination in gene therapy and cell-based medicines, notably regenerative medicine. The use of viral vectors in these treatments to introduce genetic material into cells raises the possibility of viral contamination. Thus, pharmaceutical and biotech firms heavily use viral clearance techniques to ensure the security and effectiveness of these therapies. Pharma and biotech businesses also concentrate on product development and commercialization while maintaining viral safety.
The contract research organizations segment is expected to grow at a significant rate in the market during the forecast period. CROs are extensively aware of testing procedures, virus clearance technology, and regulatory compliance. Pharmaceutical businesses can access technical expertise and knowledge from CROs they might not otherwise have. Global regulatory agencies demand CROs to manage extensive documentation, audits, and continuous viral safety testing. CROs are skilled in these. Because of their expertise, biopharmaceutical businesses choose them as their preferred partner when they want to achieve compliance criteria without having to develop internal resources.
Regional Insights
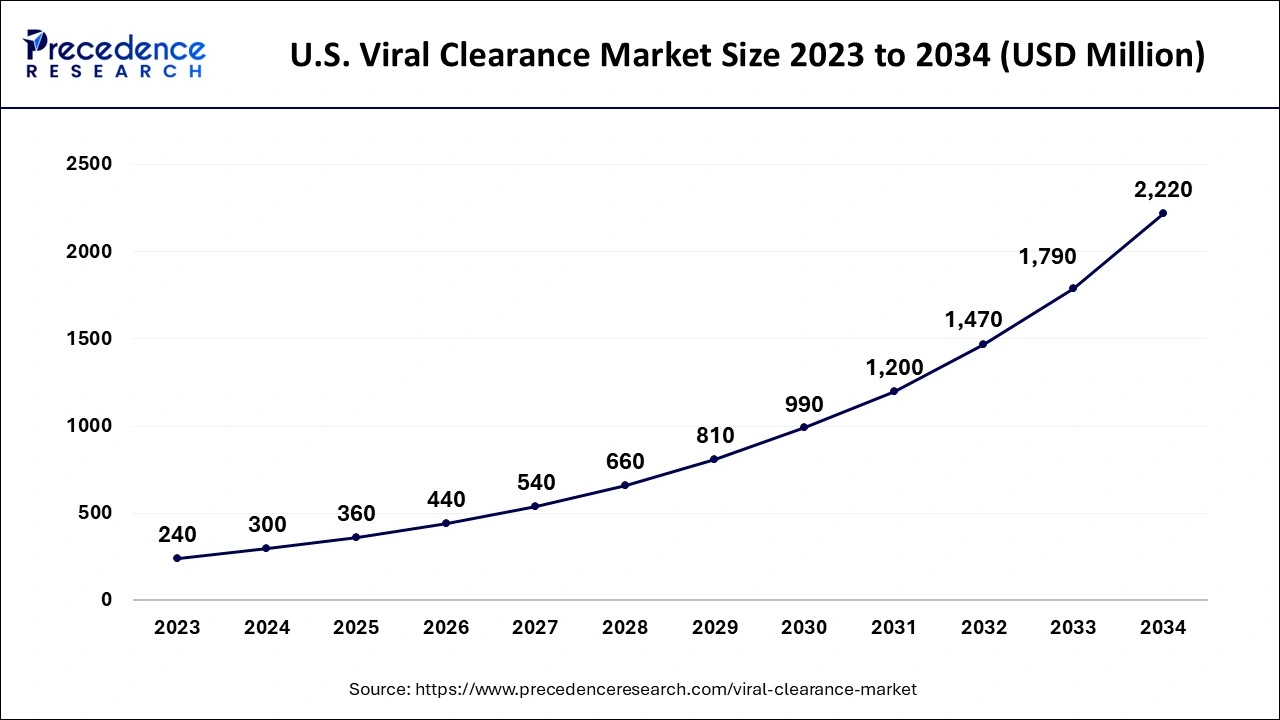
What is the U.S. Viral Clearance Market Size?
The U.S. viral clearance market size was evaluated at USD 360 million in 2025 and is projected to be worth around USD 2,576.67 million by 2035, growing at a healthy CAGR of 21.75% from 2026 to 2035.
North America dominated the viral clearance market in 2024. North America's supremacy has benefited immensely from the work of respectable regulatory agencies such as the U.S. Food and Drug Administration (FDA). These organizations set stringent standards for developing, testing, and manufacturing biopharmaceutical products. The increasing demand for biologics, such as monoclonal antibodies, cell therapies, and recombinant proteins, contributed to the regional market growth. The COVID-19 pandemic also raised attention toward the value of immunizations, increasing the demand for viral clearance services.
U.S Viral Clearance Market Analysis
The U.S. market is dominant because it has well well-developed biopharmaceutical industry, well well-developed regulatory environment, and a great volume of biologics production. The fact that the largest players on the market, like Thermo Fisher Scientific, Merck, and Sartorius, exist promotes the constant innovation of the filtration and validation technologies. The FDA's attention to viral safety in monoclonal antibodies, vaccines, and gene therapies further puts pressure on the demand.
Asia Pacific is expected to witness the fastest growth during the forecast period. This is due to the availability of a large number of CROs and the increasing production of pharmaceuticals. Many global biopharmaceutical companies are outsourcing viral clearance and other procedures from Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) in Asia Pacific due to the cost benefits of a skilled workforce and high compliance with regulatory standards. Moreover, there is a substantial increase in demand for viral clearing services in the region, thus contributing to regional market growth.
China Viral Clearance Market Analysis
China's market is rapidly expanding owing to the upsurge in the production of domestic biopharmaceuticals and government incentives for biotechnology research and development. The domestic manufacturers are now developing biosimilars, vaccines, and recombinant proteins, and this puts additional pressure on viral safety testing and clearance solutions. To meet the international standards, regulatory companies are enhancing the quality standards and promoting the use of modern filtration and validation mechanisms.
Why Is the European Viral Clearance Market Experiencing Notable Growth?
The European viral clearance market is registering with great growth due to the stringent regulatory prerequisites enshrined in the biologics safety and quality assurance by the EMA. The growing interest in monoclonal antibodies, vaccines, and advanced therapy medicinal products has led to an increase in demand for reliable viral inactivation and elimination technologies. Also, the increased outsourcing to specialized CROs and contract manufacturing organizations (CMOs) is increasing demand for services.
The UK Viral Clearance Market Trends
The UK market is characterized by effective academic-industrial cooperation and a developed life sciences environment. Growth has been fostered by the escalated biologics research, vaccine development, and clinical trials, underpinned by the forefront universities and biotech centers in Cambridge and Oxford. The Medicines and Healthcare products Regulatory Agency of the UK has stringent safety standards, and pharmaceutical companies are trying to invest in sophisticated viral clearance validation services.
Why Is the MEA Viral Clearance Market Gaining Momentum?
The Middle East and Africa viral clearance market has been on a momentum because the region is experiencing a rapidly growing biopharmaceutical industry, owing to the rise in investments in healthcare. The need to produce vaccines and biologics locally is increasing the pressure on governments to develop technologies to ensure product safety and regulatory compliance. The growth of the market is propelled by the increasing use of the latest bioprocessing methods, such as chromatography, filtration, and inactivation methods.
UAE Viral Clearance Market Trends
UAE controls the MEA viral clearance market because it has the powerful support of the government towards biotechnology and pharmaceutical production. The Vision 2030 program of Saudi Arabia emphasizes the development of drugs and vaccines within the country, which implies the growth of the demand for virus clearance validation and filtration technologies. Their leadership is further enhanced by high spending on healthcare, advanced laboratory facilities.
Why Is the Latin American Viral Clearance Market Emerging Rapidly?
The Latin American viral clearance market is fast developing as a result of the development of biopharmaceutical and vaccine production in the region. The state and commercial interests are putting much money into the local manufacturing of biologics to limit reliance on imports. Intensified regulatory systems and rising awareness of the danger of contamination by viruses in biologics are also contributing to the acceptance of viral clearance solutions.
Brazil and Mexico Viral Clearance Market Trends
The Latin American viral clearance market is dominated by Brazil and Mexico due to their developed pharmaceutical and growing biologics manufacturing capacities. Brazil has good public institutions, including Fiocruz and the Butantan Institute, which facilitate the production of vaccines and biosimilars that demand high viral safety standards. Mexico's continuous building of CROs and CMOs makes it a desirable destination for outsourced bioprocessing and clinical research.
Value Chain Analysis-Viral Clearance Market
- R&D: Initial scientific work on the development of new technologies to get viral inactivation and removal, and assays. Pre-clinically consider high-throughput procedures, filter systems, viral safety reagents, and optimization of the procedure.
Key Players: Merck KGaA, Sartorius AG, Eurofins Scientific, WuXi AppTec, Charles River Laboratories - Clinical Trials and Regulatory Approvals: Undertaking validation studies, safety testing, and regulatory filing of viral clearance assays and technologies, and new biologics and therapeutic products other requirements of the FDA/EMA.
Key Players: Charles River Laboratories, SGS SA, WuXi AppTec, Eurofins Scientific, ICON plc - Formulation and Final Dosage Preparation: The inclusion of the viral clearance procedures into the biotherapeutic formulation and final product manufacture will guarantee the removal/inactivation of the viral contaminants in the drug substance and dosage forms.
Key Players: Catalent Inc., Lonza Group, Thermo Fisher Scientific, MilliporeSigma, Sartorius AG - Packaging and Serialization: Final product packaging incorporates serialization/traceability, as well as provides integrity of sterile biologics; packaging partners also serve in the support of the cold-chain of virus-sensitive therapeutics.
Key Players: Catalent Inc., Thermo Fisher Scientific, Asahi Kasei, GE Healthcare, Lonza Group - Patient Support & Services: After-market care such as adverse event reporting, therapy surveillance, safe biologic use education, reimbursement assistance, and feedback mechanisms to manufacturers to engage in constant improvement.
Key Players: Charles River Laboratories, Eurofins Scientific, IQVIA, Syneos Health, Parexel
Viral Clearance Market Companies

- Merck KGaA
- Vironova
- Kedrion
- Sartorius AG
- Syngene International Limited
- Microbiologics
- Clean Cells
- ViruSure GmbH
- Charles River Laboratories
- WuXi Biologics
- Texcell
Recent Developments
- In August 2024, Apple Tree Partners (ATP) introduced Red Queen Therapeutics, a clinical-stage biotechnology company that focuses on creating innovative antiviral treatments for the public and immunocompromised patients who are more vulnerable to severe illness or death from common and emerging viral pathogens.
- In May 2024, a leading science and technology company, Merck, signed an agreement to acquire Mirus Bio, a specialist in the development and commercialization of transfection reagents. This acquisition will enhance Merck's offering in the viral vector manufacturing industry.
Segments covered in the report
By Method
- Viral Removal
- Type
- Chromatography
- Nanofiltration
- Precipitation
- Type
- Viral Inactivation
- Type
- Low pH
- Solvent Detergent Method
- Heat Pasteurization
- Other Viral Inactivation Methods
- Type
By Application
- Recombinant Proteins
- Blood and Blood Products
- Cellular and Gene Therapy Products
- Vaccine
- Others
By End-user
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations
- Academic Research Institutes
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Tags
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting