Viral Vectors for Non-human Primates Market Size and Forecast 2025 to 2034
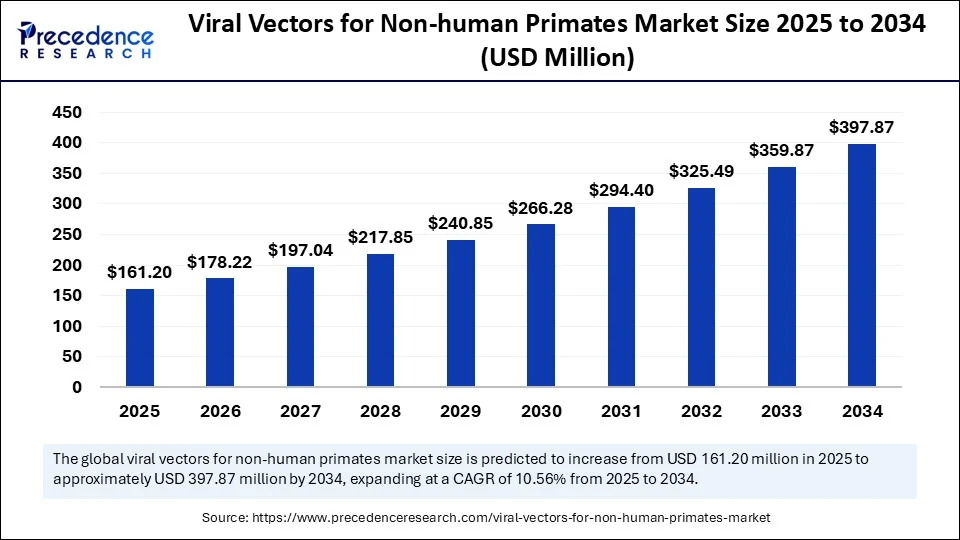
The global viral vectors for non-human primates market size accounted for USD 145.8 million in 2024 and is predicted to increase from USD 161.2 million in 2025 to approximately USD 397.87 million by 2034, expanding at a CAGR of 10.56% from 2025 to 2034. The increasing demand for novel gene therapies and the growing number of clinical studies are driving the growth of the market.
Viral Vectors for Non-human Primates Market Key Takeaways
- The global viral vectors for non-human primates market was valued at USD 145.8 million in 2024.
- It is projected to reach USD 397.87 million by 2034.
- The market is expected to grow at a CAGR of 8 10.56% from 2025 to 2034.
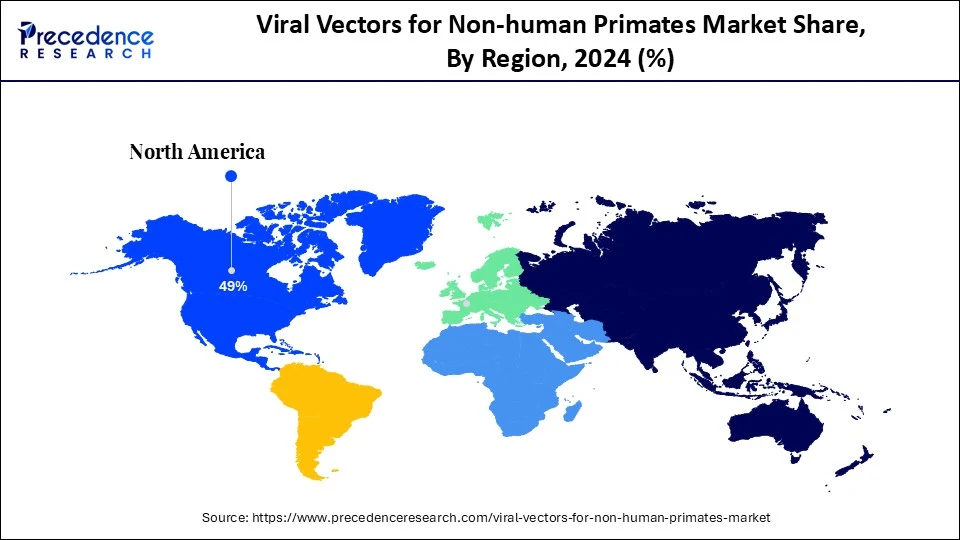
- North America dominated the global viral vectors for non-human primates market with the largest revenue share of 49% in 2024.
- Asia Pacific is expected to grow at the fastest CAGR from 2025 to 2034.
- By vector type, the adeno-associated vectors segment held a major market share in 2024.
- By vector type, the retroviral vectors segment is expected to grow at the highest CAGR between 2025 and 2034.
- By type of non-human primates, the rhesus macaques segment captured the biggest market share in 2024.
- By type of non-human primates, the cynomolgus monkey segment is expected to grow at the fastest CAGR during the forecast period.
- By application, the gene therapy segment led the market in 2024.
- By application, the vaccine research segment is expected to grow at a significant CAGR in the coming years.
- By therapeutic area, the oncological disorders segment contributed the major market share in 2024.
- By therapeutic area, the genetic disorders segment is expected to expand at the highest CAGR between 2025 and 2034.
- By end-user, the pharmaceutical and biotechnology companies segment contributed the largest market share in 2024.
- By end-user, the academic and research institutes segment is expected to expand at the fastest CAGR during the projection period.
AI Impact on the Viral Vectors for Non-human Primates Market
Artificial intelligence (AI) is transforming viral vector research in non-human primates. AI enables the development of safe, efficient, and targeted gene therapies and accelerates the development of novel therapies. AI algorithm can optimize vector design and engineering, allowing efficient gene delivery. AI enables predictive modeling and data analysis, enabling researchers to identify patterns & trends and predict potential outcomes of the trials. Machine learning (ML) models are being used to predict and mitigate potential immune responses to viral vectors in non-human primates, enhancing the safety and efficiency measures of gene therapies. The use of AI-driven modeling tools has increased in CRISPR gene editing, AAV Capsid engineering, and the prediction of vector behavior.
U.S. Viral Vectors for Non-human Primates Market Size and Growth 2025 to 2034
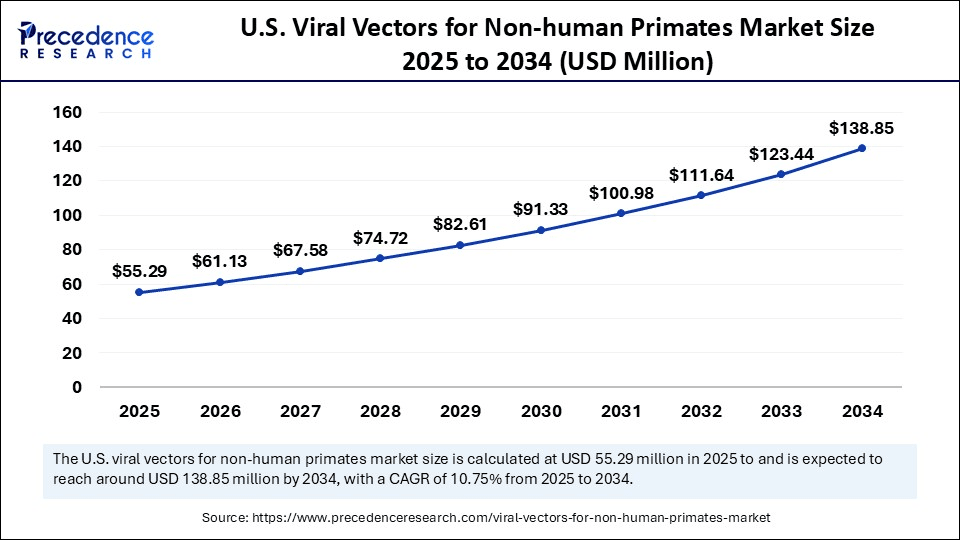
The U.S. viral vectors for non-human primates market size was exhibited at USD 50.01 million in 2024 and is projected to be worth around USD 138.85 million by 2034, growing at a CAGR of 10.75% from 2025 to 2034.
What Factors Contribute to North America's Dominance in the Viral Vector for Non-human Primates Market?
North America dominated the global viral vector for non-human primates market by holding the largest revenue share in 2024. This is mainly due to its robust research infrastructure. There is an increased pipeline for gene therapy, prompting clinical trials to ensure the efficacy of therapy for particular diseases. Advanced research facilities and the presence of a large number of market players bolstered the growth of the market. Additionally, the region is home to some of the leading pharmaceutical companies that are investing heavily in novel gene therapy research, driving the demand for viral vectors. Rising approvals for novel gene therapies, along with the increased demand for gene therapy and cell therapy, are contributing to market growth.
The U.S. is a major player in the North American viral vectors for non-human primates market. The growth of the market in the U.S. is driven by increased preclinical research and a strong focus on gene therapy research. Research on rhesus macaques has been increasing in the country due to their genetic similarities to humans. With the increasing prevalence of various life-threatening diseases like heart disease, cancer, neurodegenerative disorders, and other genetic diseases, there is a strong need for novel vaccines. Collaborative approaches among researchers and pharmaceutical & biotechnology companies bring significant opportunities for the production of viral vectors for non-human primates in the U.S.
Asia Pacific Viral Vectors for Non-human Primates Market
Asia Pacific is expected to expand at the fastest CAGR in the upcoming period, driven by rising research and development in gene therapies and vaccines. Governments of various Asian countries are investing in pharmaceutical companies to enhance self-sufficiency in biotech manufacturing. Additionally, government support for local and start-up pharmaceutical companies is fostering market growth. Ongoing collaborations between research institutions and biopharmaceutical companies, particularly in countries like China, Japan, India, and South Korea, are bringing novel approaches in the development of innovative vaccines and gene therapies.
China is a major country leading the regional market, driven by government investments in biopharmaceutical companies, favorable regulatory policies for novel research and developments, and the existence of robust contract development and manufacturing organizations (CDMOs), like Wuxi App Tech, ASKBio, and GenScript. These CDMOs have established sustainable GMP-certified production facilities of viral vectors in China.
European Viral Vectors for Non-human Primates Market
Europe is a significant player in the global market. The growth of the market in the region is attributed to the growing use of non-human primates as preclinical models for crucial disease studies, like cancer, neurological disorders, and genetic disorders. Europe has approved numerous gene therapies, driving demand for viral vectors for the clinical and commercial sectors. The ongoing shift of pharmaceutical and biopharmaceutical companies toward in-house manufacturing has increased investments in companies' own viral vectors' manufacturing capabilities to reduce dependency on CDMOs.
Germany is emerging as a major player in the market due to its robust pharmaceutical and biotechnology companies, higher number of gene therapy clinical trials, and strong viral vector production infrastructure. Germany is dominating the gene therapy clinical trial sector. There is a high demand for gene therapies. Thus, government and public entities are making efforts to boost the production of gene therapies, significantly driving market growth.
Market Overview
The global viral vectors for non-human primates market is expanding rapidly due to the rising use of non-human primates in preclinical studies, driven by increased demand for vaccines and gene therapies for human trials. The rising prevalence of genetic disorders, cancer, and neurological disorders is boosting the demand for advanced gene therapies, regenerative medicine, and vaccine developments. Research institutes and biopharmaceutical companies are collaborating in areas to enhance the production of efficient and safe gene therapies. Large companies are investing in their in-house manufacturing of advanced viral vectors for non-human primates. Additionally, governments worldwide have increased investments in research and development, supporting market growth. The rising production of specific vector types, like Adeno-associated virus vectors, contributes to market expansion. These vectors are mainly being used in clinical trials and FDA-approved applications due to their high safety and efficiency.
What are the Key Trends of the Viral Vectors for Non-human Primates Market?
- Prevalence of Genetic Disorders: The increased prevalence of genetic disorders, infectious diseases, and cancers is driving demand for the production of viral vectors to use in non-human clinical trials.
- Increased Clinical Studies: The growing number of clinical studies in gene therapies is driving the need for high-quality viral vectors.
- Regulatory Approvals: Regulatory frameworks are the approval process of novel gene therapies, empowering the production of viral vectors for non-human primates research.
- Collaborative Research: Ongoing collaborations between research institutions and pharmaceutical & biopharmaceutical companies are enabling the development of standardized, high-quality, and high-performance viral vectors for non-human primates' research.
- Technological Advancements: Advancements in gene therapies, like CRISPR integration and AAV-based vectors, improve viral vectors' efficacy and precision.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 397.87 Million |
| Market Size in 2025 | USD 161.2 Million |
| Market Size in 2024 | USD 145.8 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 10.56% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Vector Type, Type of Non-human Primates, Application, Therapeutic Area, End-user, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Rising Preclinical Studies
The rising number of preclinical studies in non-human primates is driving significant approaches in gene therapy. The increased number of gene therapy research studies, driven by the growing prevalence of genetic disorders, is driving the need for viral vectors for non-human primates. These vectors enable the safety and efficacy of new gene therapies in non-human trials. The increased prevalence of cancer, neurology, and rare genetic disorders is the major factor driving the need for novel gene therapies. Additionally, ongoing advancements in gene editing technologies like CRISPR are improving the efficiency and precision of these viral vectors.
Restraint
High Production Cost
The high cost associated with the production of viral vectors, particularly at the scale required for non-human primate research and therapeutics, is a major factor restraining the growth of the viral vectors for non-human primates market. The manufacturing process of viral vectors is complex, leading to higher production costs. Additionally, the high cost of raw materials like reagents, cells, and plasmids adds to production costs. Scale-up challenges like increasing viral vector volume, along with quality and consistency, become difficult and add more cost. Moreover, regulatory compliance and requirements for producing viral vectors increased time consumption and cost. The rigorous quality control process to ensure the safety and efficacy of viral vectors can be costly, increasing the final cost of vectors.
Opportunity
Technological Innovations
Technological innovations like CRISPR gene editing, AVV vectors, transient transfection technology, single-use technologies, and novel vector engineering, like mosaic, combinatorial vector libraries, and chimeric, are significantly pushing market growth further. Breakthrough gene editing technologies like CRISPR are enhancing the efficiency and precision of viral vectors. Transient transfection technology enables the large-scale production of viral vectors. Single-use technologies are facilitating enhanced productivity and flexibility in vector production. AVV vectors are being used in preclinical studies for more transparency and triptolide. Additionally, novel vector engineering enables high efficiency, specialty, and safety of viral vectors for non-human primates.
Vector Type Insights
Which Vector Type Segment Dominated the Viral Vectors for Non-human Primates Market in 2024?
The adeno-associated vectors segment dominated the market with the largest share in 2024 due to the increased focus of researchers on adeno-associated vectors (AAV) for gene therapy. These vectors are widely being used in gene therapy research for non-human primates, driven by their broad tissue tropism and ability to sustain long-term transgene expression. The increased prevalence of genetic disorders and infectious diseases has increased the use of adeno-associated vectors, bolstering segmental growth.
The retroviral vectors segment is expected to grow at the highest CAGR during the forecast period, driven by their widespread use in gene therapy and treatments of various diseases. Retroviral vectors can integrate into the host cell's genome, enabling stable gene expression. The ability of these vectors to enhance efficiency, safety, and tropism makes them suitable for use in gene therapy research and therapeutic areas like vaccine development.
Type of Non-human Primates Insights
What Made Rhesus Macaques the Dominant Segment in the Viral Vectors for Non-human Primates Market?
The rhesus macaques segment dominated the market with a major share in 2024. This is mainly due to their genetic similarities to humans, which make them suitable for use in research of human diseases. Rhesus macaques match approximately 93% DNA of human beings, which drives their wide adoption for gene therapy research, vaccine developments, and for better understanding of pathogenesis diseases. The high genetic similarities to humans make rhesus macaques ideal primates for modeling human disease and gene therapy testing. A growing number of clinical trials are increasing the use of rhesus macaques, further enhancing the segment's growth.
The cynomolgus monkey segment is expected to grow at the fastest rate during the forecast period. The growth of the segment is attributed to the rising use of cynomolgus macaque in gene therapy research and vaccine developments. Cynomolgus is mostly used in biomedical research due to its similarities to human genetics and physiology. The cynomolgus macaque has been used as a crucial model in human disease studies and the development of novel therapies.
Application Insights
How Does the Gene Therapy Segment Dominate the Market in 2024?
The gene therapy segment dominated the viral vectors for non-human primates market with the biggest share in 2024 due to increased research and development in gene therapy and the wide use of viral vectors for gene therapy in non-human primates. Non-human primates play a vital role in preclinical research because of their physiological and genetic similarities to humans. Viral vectors deliver therapeutic genes in non-human primates. Viral vectors are crucial in gene therapy research using non-human primates.
The vaccine research segment is expected to grow at the fastest rate over the projection period. Viral vectors enable researchers to test vaccines in animal models before their use in human clinical trials. Non-human primates are similar to humans in genetics and are used as preclinical models for targeting vaccines, helping with the development of novel vaccines. The rising use of viral vectors in vaccine preclinical research is fostering segment growth.
Therapeutic Area Insights
How Does the Oncological Disorders Segment Dominate the Viral Vectors for Non-human Primates Market in 2024?
The oncological disorders segment dominated the market by holding a significant share in 2024. This is mainly due to the increased prevalence of cancer, leading to high demand for efficient and safe innovative treatments. With the increased cancer burden worldwide, the number of clinical trials and the development of novel vaccines, particularly in oncolytic viruses, has risen. This created the need for viral vector research in non-human primates. Moreover, the growing need for preclinical research in cancer therapies ensures the segment's long-term growth.
The genetic disorders segment is expected to expand at the highest CAGR in the upcoming period, driven by the wide use of non-human primates in preclinical gene therapy research. The prevalence of genetic disorders has increased worldwide, driving the demand for gene therapies. The increased gene therapy research and use of non-human primates, due to their genetic and physiological similarities to humans, are fostering segment growth.
End-user Insights
Which End-user Segment Dominate the Viral Vectors for Non-human Primates Market?
The pharmaceutical and biotechnology companies segment dominated the market with the maximum share in 2024 and is likely to sustain its growth trajectory over the forecast period, driven by increased research and development of novel treatments and medicines. Pharmaceutical and biotech companies are investing heavily in research and development in areas like neurodegenerative disease and genetic disorders. Growing gene therapy research by pharmaceutical and biotechnology companies is increasing the demand for viral vectors suitable for non-human primates. These companies are focusing on developing advanced viral vectors, bolstering segmental growth.
The academic and research institutes segment is expected to expand at the fastest CAGR during the projection period due to the growing research on neuroscience and gene therapies. Non-human primates are often used in preclinical studies and neuroscience research. These institutes actively engage in research, driving the demand for non-human primates for more efficient and safety of therapies to use before human trials. Additionally, rising government investments to modernize research infrastructure fuels the segment's growth.
Viral Vectors for Non-human Primates Market Companies

- Merck KGaA
- 4D Molecular Therapeutics
- Barinthus Biotherapeutics
- Biovian Oy
- Andelyn Biosciences
- Lonza Group AG
- Fujifilm Diosynth Biotechnologies
- CRISPR Therapeutics
- Oxford Biomedica
- REGENXBIO Inc.
Latest Announcement by Industry Leader
At the American Society of Gene & Cell Therapy (ASGCT) 28th Annual Meeting, held May 13 to 17, 2024, in New Orleans, LA, Nicholas Giovannone, PhD, a senior principal scientist at Regeneron, gave a presentation entitled “Successful AAV vector re-administration via two distinct B cell immunomodulation strategies in non-human primates.” The presentation covers a comparison between a novel strategy using bispecific antibodies and a more traditional strategy using rituximab. He emphasized that nonhuman primate methods are transient, aiming to maintain a seronegative state only during key treatment windows, potentially enabling flexible and repeatable gene therapy.
Recent Development
- In February 2025, researchers at Oregon Health & Science University (OHSU) developed the first transgenic nonhuman primate model for hepatitis B virus study. The model includes a rhesus macaque, genetically modified to express the human NITCP protein. The model will help researchers to innovate and develop treatments for hepatitis B.
(Source: https://medicalxpress.com)
Segment Covered in the Report
By Vector Type
- Adenoviral Vectors
- Adeno-associated Vectors
- Retroviral Vectors
- Lentiviral Vectors
- Others
By Type of Non-human Primates
- Marmosets
- Rhesus Macaques
- Cynomolgus Monkey
- Others
By Application
- Gene Therapy
- Vaccine Research
By Therapeutic Area
- Genetic Disorders
- Infectious Diseases
- Oncological Disorders
- Others
By End-user
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting