Viral Vectors-Based Gene Therapy for Non-Human Primates Market Size and Forecast 2025 to 2034
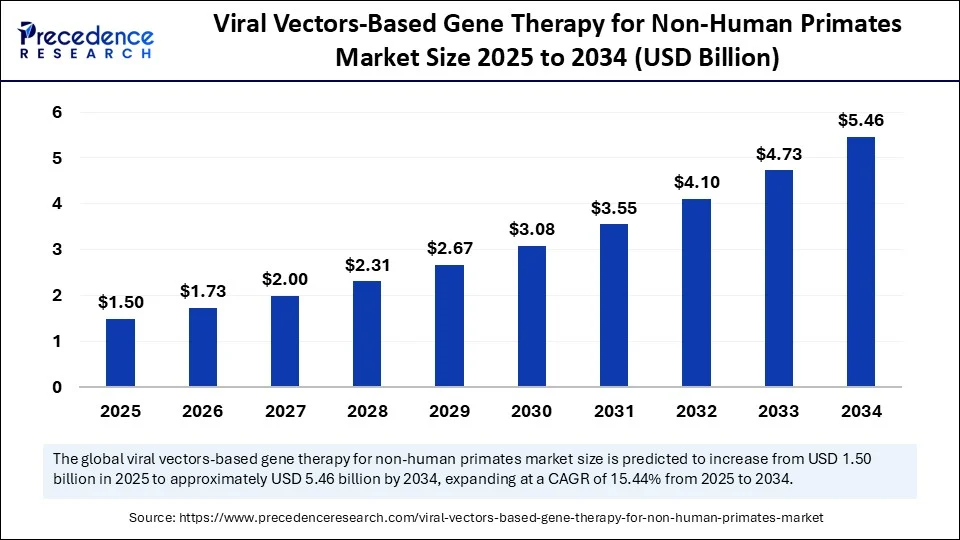
The global viral vectors based gene therapy for non-human primates market size accounted for USD 1.3 billion in 2024 and is predicted to increase from USD 1.5 billion in 2025 to approximately USD 5.46 billion by 2034, expanding at a CAGR of 15.44% from 2025 to 2034. It is expected to grow at a compound annual growth rate (CAGR) of xx% from 2025 to 2034. The increased prevalence of genetic disease and demand for personalized medicines are driving the growth of the market.
Viral Vectors-Based Gene Therapy for Non-Human Primates MarketKey Takeaways
- In terms of revenue, the global viral vectors-based gene therapy for non-human primates market was valued at USD 1.3 billion in 2024.
- It is projected to reach USD 5.46 billion by 2034.
- The market is expected to grow at a CAGR of 15.44% from 2025 to 2034.
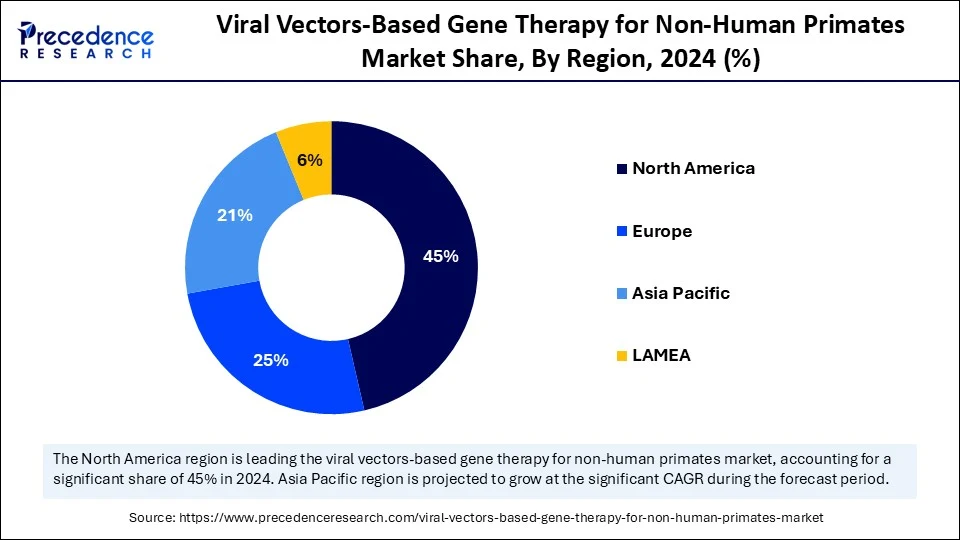
- North America dominated the global viral vectors-based gene therapy for non-human primates market with the largest share of 45% in 2024.
- Europe is expected to grow at a significant CAGR of approximately 25% from 2025 to 2034.
- By vector type, the adeno-associated virus (AAV) segment captured the biggest market share of 55% in 2024.
- By vector type, the lentiviral vectors segment will grow at an approximate 20% CAGR between 2025 and 2034.
- By application, the ophthalmology (retinal gene therapy) segment contributed the largest market share of 30% in 2024.
- By application, the CNS & neurological disease models segment will expand at a CAGR of 20% between 2025 and 2034.
- By service type, the vector manufacturing & GMP-grade supply segment held the major market share of 60% in 2024.
- By service type, the immunogenicity & safety assays segment is expected to grow at the fastest CAGR between 2025 and 2034.
- By end-user, the biopharma & gene therapy companies segment generated the highest market share of 50% in 2024.
- By end-user, the preclinical CROs segment will expand at a significant CAGR between 2025 and 2034.
How is AI Revolutionize the Viral Vectors-Based Gene Therapy for Non-Human Primates Studies?
Artificial Intelligenceis playing a revolutionary role in viral vector-based gene therapy for non-human primates studies by optimizing vector development, custom design, delivery, and safety assessments. AI is capable of analyzing a complex and broad range of data from gene therapy trials by detecting various patterns, enabling quick and informed decision-making in treatment. Predictive modeling of AI optimizes vector performance, safety, and efficacy, enabling the development of better vector designs.
The rising demand for personalized medicines is making AI a crucial model in research & development, even in preclinical studies. AI is a tailored tool that helps to develop gene therapies according to individual patients. AI has proven its significant role in gene editing and advancements. However, the AI algorithms in designing and optimizing viral vectors for specific applications, as well as further improving gene delivery and expression, make it vital in non-human primate research studies.
U.S. Viral Vectors-Based Gene Therapy for Non-Human Primates Market Size and Growth 2025 to 2034
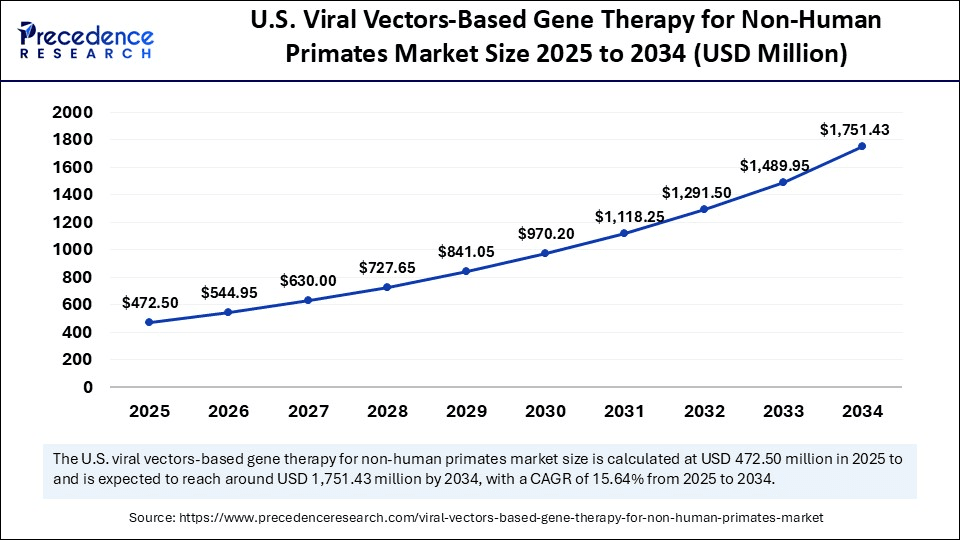
The U.S. viral vectors based gene therapy for non-human primates market size was exhibited at USD 409.50 million in 2024 and is projected to be worth around USD 1,751.43 million by 2034, growing at a CAGR of 15.64% from 2025 to 2034.
What Made North America the Dominant Region in the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
North America dominated the global viral vectors-based gene therapy for non-human primates market while holding the largest share in 2024. This is primarily due to its well-established research institutions and government support. There is a high prevalence of genetic disorders and demand for personalized medicines, driving the need for viral vectors. The rising emphasis on optimizing and producing viral vectors, including adeno-associated virus (AAV) and lentiviral vectors, is driving significant innovation in the emerging market. Additionally, significant focus on research for using viral vectors for the rising diseases, like genetic disorders, oncological disorders, and neurodegenerative diseases, is driving the adoption of viral vector-based gene therapy for non-human primates clinical trials.
The U.S. is a major player in the regional market, contributing to growth due to its advanced research infrastructure, the growing prevalence of genetic disorders, demand for personalized medicines, and strong funding from both government and private organizations for gene therapy research. The U.S. boasts a well-developed biotech industry, with a strong presence of several key market companies and their efforts in the development of viral vector-based gene therapies, supporting market growth.
For instance, government agencies in the U.S., like the National Institutes of Health (NIH), provide funding and regulatory support for viral vector-based gene therapy innovations and developments.
Europe Viral Vectors-Based Gene Therapy for Non-Human Primates Market Trends
Europe is emerging as the fastest-growing region, driven by the increased prevalence of genetic and oncological disorders. The demand for non-human primates (NHPs) in preclinical testing. Europe is home to a well-established research infrastructure. The expanding emphasis on the development and application of gene-modified cell therapies, as well as the growing use of viral vectors in clinical trials, is driving demand for innovative and customized viral vectors. Europe's advanced biotech industry is at the forefront of the development of cutting-edge gene therapies. Ongoing collaborations between biotech companies, academia, industry, government, and research initiatives are driving significant innovations in R&D.
Germany is a significant market. The country's advanced research & development infrastructure, growing numbers of clinical trials, and strong government initiatives support market growth. The European Medicines Agency (EMA) is committed to providing clear regulatory approval and guidelines for gene therapy innovations. Germany's strong manufacturing capabilities for plasmid DNA and viral vectors have led to significant innovations and developments in gene therapy. The expansion of biotech companies, the setup of facilities, and government support are further contributing to market growth.
Asia Pacific Viral Vectors-Based Gene Therapy for Non-Human Primates Market Trends
Asia Pacific is expected to experience notable growth in the coming years, driven by ongoing advancements in gene therapy techniques and growing research for genetic disorders and therapies for complex diseases. The demand for gene therapies has increased in the Asia Pacific, driving demand for non-human primates in preclinical testing. Additionally, advancements in the biotechnology industry are driving innovations and the development of novel viral vectors and gene therapies. The government's investments in local pharmaceutical industries and research and development (R&D) foster this growth.
China is a major player in the regional market, contributing to growth due to its favorable regulatory environment, strong presence of major contract development and manufacturing organizations (CDMOs), and government investments in gene therapy infrastructure. China's significant progress in advancing and upgrading its regulatory frameworks for cell-based research activities is further contributing to the advancement of gene therapy developments.
Market Overview
The viral vectors-based gene therapy for non-human primates market includes the design, production, and supply of viral vectors (e.g., AAV, lentivirus, adenovirus, retrovirus) used in preclinical gene therapy and viral delivery studies in non-human primates, particularly rhesus macaques and cynomolgus monkeys. NHPs serve as critical translational models for evaluating safety, tropism, efficacy, and immune response of novel gene therapies and CRISPR-based interventions before human trials. The growing innovations and developments of AAV vectors and CRISPR gene editing are driving the precision and effectiveness of gene delivery.
The market expansion is further driving innovation in gene therapy advancements, as well as an increase in CDMO outsourcing to enhance capacity and meet growing demand, and the adoption of preclinical studies. Government funding in R&D for viral vector manufacturing and gene therapy research is further contributing to the growing adoption of preclinical research for the use of viral vector-based gene therapy in non-human primates.
- In February 2025, the 3rd Viral Vector Process Development and Manufacturing Summit was held in Boston to bring together industry leaders from Sanofi, Kite, and Ultragenyx for transformative discussions on viral vector production for cell and gene therapies. (Source: https://biopharma-asia.com)
What are the Key Trends in the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- Genetic Disease Prevalence: The growing prevalence of genetic disease has increased demand for effective and safe therapies, including viral vector-based gene therapy.
- Demand for Personalized Medicines: The demand for personalized medicines has increased, driving innovations and developments in gene therapies tailored to individual genetic profiles, thereby increasing the need for non-human primate-based clinical trials.
- Technological Advancements: The advancements in gene editing technologies are bringing enhancements in the precision of gene delivery, driving innovations in genetic disease treatments.
- Strategic Collaborations: Pharmaceutical companies, contract research organizations (CROs), research institutions, and academic institutions are collaborating to enhance research and development, thereby contributing to the adoption of preclinical research.
- Regulatory Support: The increasing regulatory support and approvals for testing viral vector-based gene therapies in non-human primates, along with clearer guidelines, are driving the market.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 5.46 Billion |
| Market Size in 2025 | USD 1.5 Billion |
| Market Size in 2024 | USD 1.3 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 15.44% |
| Dominating Region | North America |
| Fastest Growing Region | Europe |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Vector Type, Application, Service Type, End User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising Preclinical Research
The increasing number of preclinical research activities, particularly in the study of genetic disorders and the development of innovative therapies, drives the market's growth. Non-human primates share genetic and physiological similarities with humans, making them a crucial model for gene therapy research and development. The growing prevalence of genetic disease is driving the need for effective treatments. The rising advancements in gene therapy for beautiful disease are fueling research in the pipelines. Additionally, advancements in manufacturing processes and technologies, such as single-use bioreactors and suspension-adapted cell lines, are enabling the large-scale production of viral vectors, contributing to the growing adoption of preclinical research studies.
Restraint
High Cost
The high costs associated with vector production, clinical trials, and navigating regulatory challenges hinder the growth of the viral vectors-based gene therapy market for non-human primates. The production of viral vector-based gene therapies needs significant investments in research, development, and testing. The development of customized vector design and optimization requires resources and expertise, which adds to the cost. The complete production process of high-quality viral vectors requires specialized equipment, expertise, and facilities. Additionally, the high costs associated with regulatory compliance and ethical concerns further add to the burden of developing and commercializing viral vector-based gene therapies.
Opportunity
Advancements in Gene Therapy
The growing prevalence of genetic disorders and increasing demand for personalized medicines have fueled advancements in gene editing technologies, such as CRISPR-Cas9, and boosted investments in gene therapy research. The growing adoption of gene therapy-based research drives the need for non-human primates (NHPs), as they are crucial preclinical models that help to evaluate the safety and efficacy of novel gene therapies before human trials. Additionally, growing advancements in viral vector technologies, such as AAV and lentiviral vectors, lead to more precise and efficient delivery of genetic materials. Advancements in gene therapy and growth in research & development for the development of novel and innovative gene therapies for multiple diseases and disorders are contributing to the adoption of viral vectors for non-human primates.
Which Vector Type Segment Dominate the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
The adeno-associated virus (AAV) segment dominated the market with the largest share in 2024. This is primarily due to its widespread application in preclinical testing, driven by its excellent safety and efficiency profiles, as well as its long-term gene expression capabilities. The adeno-associated virus (AAV) exhibits broad tissue tropism, making it a highly preferred choice for use in clinical trials. Adeno-associated virus (AAV)- based therapies, such as Zogenix and Luxturna, are gaining traction in gene therapy. The use of adeno-associated virus (AAV) is high in retinal gene transfer and the treatment of central nervous system diseases, hemophilia, and ocular conditions. The adeno-associated virus (AAV) has been gaining large FDA approvals due to its good safety profile and versatility in manufacturing. The rising focus on AAV genome regulations, with an emphasis on inverted terminal repeats (ITRs) and AAV capsid-genome interactions, further ensures the long-term growth of this segment.
The lentiviral vectors segment is expected to grow at the fastest CAGR in the upcoming period due to their ability to transduce dividing and non-dividing cells. Lentiviral vectors integrate into the host genome for long-term genetic expression and low immunogenicity, making them the preferred choice for gene delivery in non-human primates. This vector offers unique research advantages and potential therapeutic applications. Lentiviral vectors can be engineered for target-specific cell types, making them versatile in gene therapy. The need for efficient gene delivery drives demand for lentiviral vectors.
Application Insights
What Made Ophthalmology the Dominant Segment in the Viral Vectors-Based Gene Therapy for Non-Human Primates Market in 2024?
The ophthalmology (retinal gene therapy)segment dominated the market while holding the largest share in 2024. This is mainly due to the widespread use of viral vector-based gene therapy in treating various ocular diseases, such as retinal disorders and age-related macular degeneration. The use of viral vectors, particularly AAV, has increased in retinal gene therapy due to their large success rates in preclinical studies using non-human primates. AAV-based gene therapy used in clinical trials for retinal disease has demonstrated efficient safety and tolerability. Gene therapy is crucial in treating genetic disorders that affect the retina, such as Leber congenital amaurosis. The growing cases of age-related macular degeneration are driving demand for gene therapies.
The CNS & neurological disease models segment is expected to grow at the fastest rate over the forecast period, driven by curative benefits of viral vectors in disease mechanisms, therapeutic efficacy, and genetic delivery strategies. Parkinson's and Huntington's. The use of viral vectors has increased for the development of gene therapies for neurological diseases like chronic pain and epilepsy. The CNS & neurological disease models are crucial for preclinical research to refine viral vector designs, safety profiles, and delivery methods. These models are crucial for understanding disease mechanisms, optimizing viral vectors, and determining the most effective delivery routes.
Service Types Insights
Why Did the Vector Manufacturing & GMP-grade Supply Segment Dominate the Viral Vectors-Based Gene Therapy for Non-Human Primates Market in 2024?
The vector manufacturing & GMP-grade supply segment dominated the market in 2024 due to the increased demand for high-quality vectors in research and preclinical studies. The vector manufacturing & GMP-grade supply play a crucial role in making viral vectors available. The demand for high-quality viral vectors, driven by increased adoption of gene therapies are contributes to demand for specialized vector manufacturing & GMP-grade supply services. Additionally, the role of vector manufacturing services in enhancing manufacturing processes and enabling efficient production makes them essential for the large-scale production of viral vectors, thereby reducing costs. The GMP-grade supply ensures compliance with regulatory standards.
The immunogenicity & safety assays segment is expected to expand at the fastest CAGR in the coming years due to their crucial role in clinical development and adoption of gene therapies. The immunogenicity assessment, including innate and adaptive immune responses, plays a crucial role in demonstrating the safety and efficacy of gene therapies in preclinical studies of non-human primates. The ongoing expansion of immunogenicity studies in non-human primates is driving the growth of this segment. Additionally, the key role of immunogenicity & safety assays in navigating regulatory landscapes and achieving clinical trial approvals is leveraging segment growth.
End-user Insights
Which End-User Segment Dominate the Viral Vectors-Based Gene Therapy for Non-Human Primates Market in 2024?
The biopharma & gene therapy companies segment dominated the market with a major revenue share in 2024. This is primarily due to their expanding pipeline for novel gene therapies and investments in R&D. The increasing prevalence of various life-threatening diseases and demand for novel and innovative treatment options have created a need for viral vectors in vaccine development. The increased demand for effective treatments, driven by genetic disorders, has further contributed to the rising impact on innovations and developments in gene therapies. Biopharma and gene therapy companies are essential in the research and development of novel therapies, including CAR-T cell therapies, oncolytic viruses, and vaccine developments, as well as driving demand for comprehensive viral vectors. The government investment in biopharma & gene therapy companies is further aiding the segment expansion.
The preclinical CROs segment is expected to grow at the highest CAGR over the forecast period, driven by their expertise in preclinical research. The preclinical CROs play a crucial role in non-human primate models to ensure the safety and efficacy of viral vector-based gene therapies before human trials. CROs hold in vivo studies in non-human primates to enhance the safety and efficacy of gene therapy products. The preclinical CROs help with the safety design and planning of preclinical studies for gene therapies. CROs do the development and testing of viral vectors for gene therapy applications. The preclinical CROs analyze and report data from preclinical studies, making it essential for human trials.
Viral Vectors-Based Gene Therapy for Non-Human Primates Market Companies

- Charles River Laboratories
- Catalent Gene Therapy (Biodextris)
- Vigene Biosciences
- GenScript ProBio
- Lonza (Capsugel)
- Thermo Fisher Scientific
- REGENXBIO
- Aldevron (Danaher)
- Novasep / Rentschler
- Oxford Biomedica
- GE Healthcare (Cytiva)
- Wave Life Sciences
- BioIVT / BioIVD
- ICON plc
- Covance (Labcorp)
- Envigo (Inotiv)
- SAB Biotherapeutics
- Precision Nanosystems
- Battery-quality NHP Centers (e.g., SNPRC)
- Inotiv (formerly BioAnalytics)
Recent Developments
- In June 2025, a research team from the University of Osaka has uncovered the molecular mechanism behind how adeno-associated virus (AAV) vectors release their therapeutic genetic material, an essential step in the effectiveness of gene therapy. The study demonstrated structural changes in the VP1 protein within AAV capsids, showing that heat induces a transformation in the protein's N-terminal regions and allows the viral genome to be injected.(Source:https://www.drugtargetreview.com)
- In February 2025, the Oregon Health & Science University researchers developed the first transgenic non-human primate model, genetically modified to carry a human gene for the study of the hepatitis B virus.(Source: https://news.ohsu.edu)
Segment Covered in the Report
By Vector Type
- Adeno-Associated Virus (AAV)
-
- Best safety profile, CNS tropism studies in macaques
- Lentiviral Vectors
- Ex vivo HSC/gene-modified cell studies
- Adenovirus (~12%)
- High payload, transient expression models
- Herpes Simplex Virus (HSV) (~6%)
- Neuronal targeting in NHP CNS
- Other (e.g., Vaccinia, Retrovirus) (~7%)
By Application
- CNS & Neurological Disease Models
- Parkinson's, Alzheimer's, spinal cord injury
- Ophthalmology (Retinal Gene Therapy)
- Cardiovascular / Muscle Gene Delivery
- Liver-Directed Gene Therapy
- Oncology (oncolytic viruses, immunotherapy vectors)
- Metabolic Disease Models
By Service Type
- Vector Manufacturing & GMP-grade Supply
- GLP Toxicology & Biodistribution Studies
- In Vivo Dosing & Surgical Delivery
- Immunogenicity & Safety Assays
- PK/PD & Transgene Expression Analytics
By End User
- Biopharma & Gene Therapy Companies
- Academic & Non-Profit Research Institutes
- Preclinical CROs
- Government & Safety-Regulatory Labs
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting