Virtual Clinical Trials Market Size and Forecast 2025 to 2034
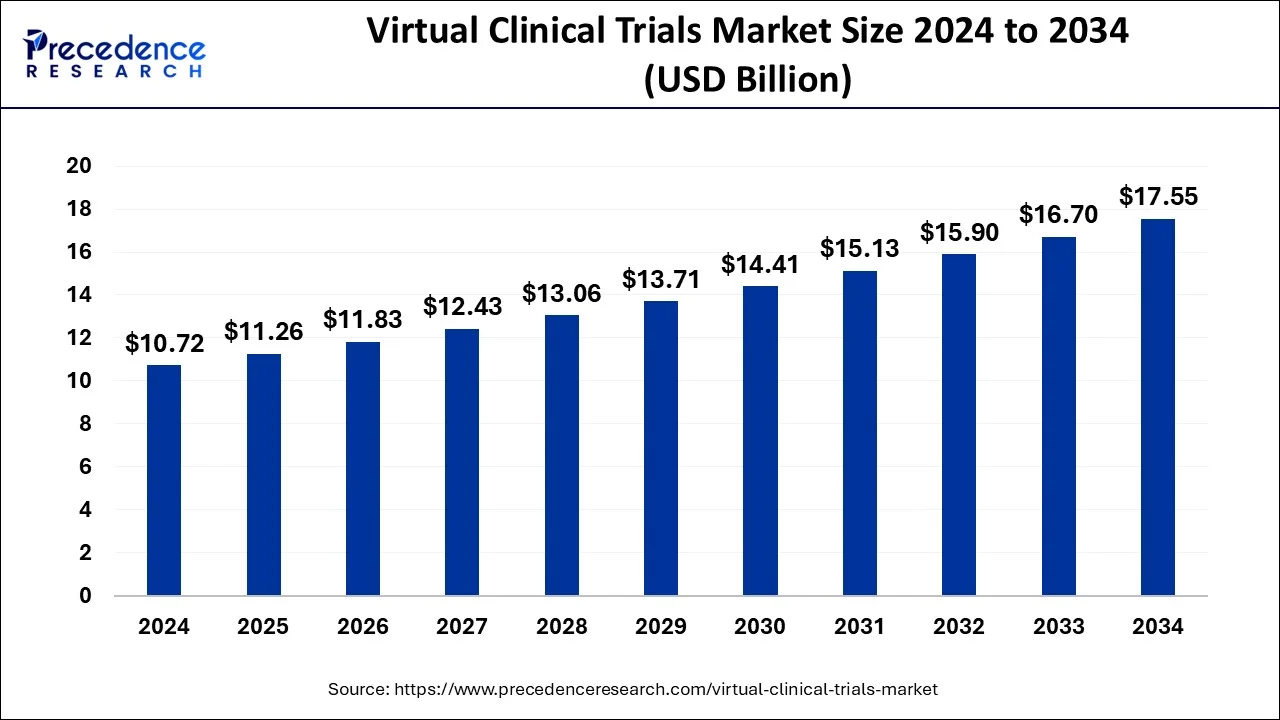
The global virtual clinical trials market size is estimated at USD 10.72 billion in 2024 and is anticipated to reach around USD 18.31 billion by 2034, expanding at a CAGR of 5.05% from 2025 to 2034. The rising digitization in the healthcare sector, increasing R&D activities, and rising adoption of digital health solutions contribute to market expansion. Rising collaborations between clinical research organizations and biotechnology companies and the rising number of clinical trials further boost the growth of the virtual clinical trials market.
Virtual Clinical Trials Market Key Takeaways
- By study design, the interventional configuration segment contributed 48% market share in 2024.
- By indication, the oncology section leads the market and accounted revenue share of around 27% in 2024.
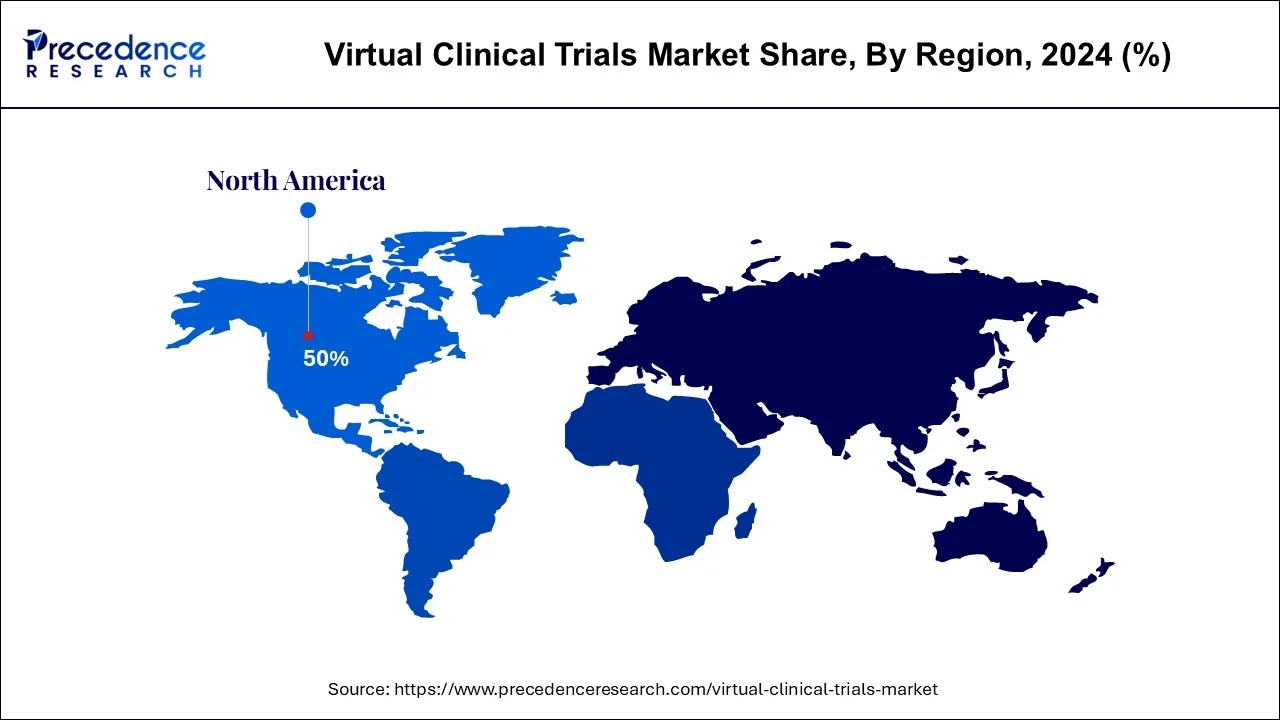
- North America led the virtual clinical trials market with the highest share in 2024.
- By region, Asia Pacific is expected to witness the highest CAGR during the forecast period.
How does AI impact the Virtual Clinical Trials Market?
Integrating artificial intelligence into virtual clinical trials redefines clinical trials by making them highly efficient, faster, and more precise than before. AI algorithms can significantly reduce time by easily analyzing health records that help identify eligible participants. With AI's help, adverse events can be detected in clinical trials at the initial phase by collecting data in real-time using wearable devices and mobile apps. Furthermore, AI has the ability to analyze large datasets, which helps identify suitable participants for trials. It also helps accelerate clinical trial processes by automating several tasks, such as data collection and processing.
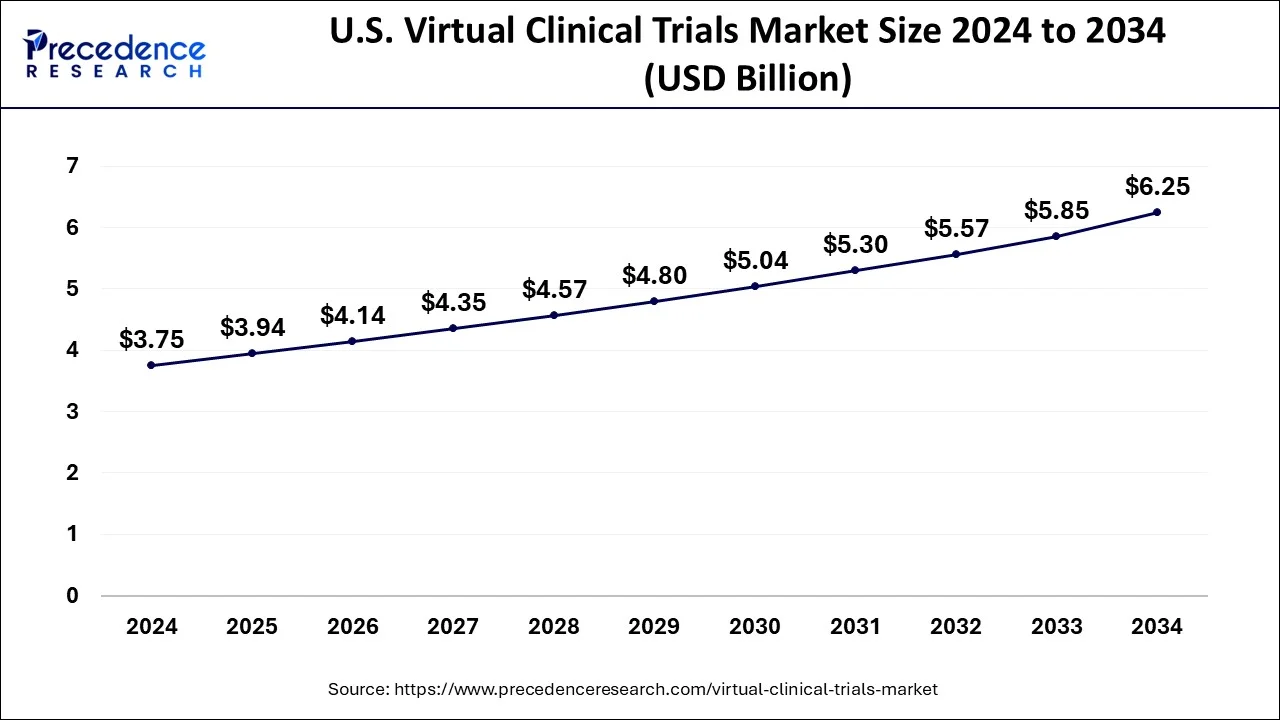
U.S. Virtual Clinical Trials Market Size and Growth 2025 to 2034
The U.S. virtual clinical trials market size is evaluated at USD 3.75 billion in 2024 and is predicted to be worth around USD 6.25 billion by 2034, rising at a CAGR of 5.24% from 2025 to 2034.
North America region dominated the virtual clinical trials market in 2024. The fragment is supposed to proceed with its predominance over the figure period. This can be credited to expanding R&D around here, expanding the reception of new advances in clinical examination as well as government support. Moreover, market players are additionally utilizing computerized advances to address client issues. For example, Parexel performed in excess of 100 decentralized trials including cross breed and virtual methodologies. Covance additionally has around 1,900 LabCorp Patient Service Centers across the U.S. that carry the preliminary to patients.On November 2019, Janssen drug auxiliary of Johnson and Johnson in a joint effort with the PRA Health Sciences, sent off computerized clinical preliminary set-up CHIEF-HF, a totally decentralize, sign chasing, portable clinical review. The organization plans to use wearable gadgets and brilliant innovation to proficiently accumulate and dissect proof for evaluating viability of Canagliflozin in the general population with cardiovascular breakdown with presence of nonattendance of type 2 diabetes.
The increased number of clinical trials in the region, especially in the U.S., further bolstered the market. According to the National Library of Medicine, as of January 2024, there were about 20,465 clinical trials recruiting patients in the U.S. In west region of U.S. that is in California there is multiple well renowned universities along with technologically well build structures that supports virtual clinical trials to launch an innovative products and procedures.
The United States leads North America's virtual clinical trials market due to its advanced technological infrastructure, a high number of clinical research organizations (CROs), and government backing for decentralized trials. The FDA is increasingly supporting the use of digital health tools and remote health monitoring after all the progress made during the COVID-19 pandemic and promoting virtual clinical trials as a viable option. Leading pharmaceutical companies, like Pfizer and Johnson & Johnson, are embracing virtual clinical trials in order to reduce patient burden and drive timeline acceleration. As the country has one of the highest rates of chronic disease, there is a large interest in remote trial models.
Asia Pacific is projected to grow at the highest CAGR during the estimate time frame inferable from the rising accessibility of a huge patient pool empowering simple enlistment of competitors alongside improved entrance of computerized advances in the locale. Besides, the flare-up of Covid is expected to support the reception of telemedicine, accordingly driving the market in the locale. Japan was the biggest market in Asia Pacific in 2020, significantly because of high medical care and R&D consumption and expanding interest for virtual clinical trials on the lookout.
Europe saw a huge development as far as CAGR in the worldwide virtual trials market throughout the course of recent years attributable to rising R&D in the medical services area and for being one of the most unfavorably impacted districts by Covid-19. In July 2020, Synairgen, a pharma organization, sent off 2 trials of their IFN-beta medication for the patients that are in danger for Covid-19, one at-home and the other extreme patients in clinic. The organization understood its interferon beta medication, SNG001, which was created for ongoing obstructive aspiratory illness (COPD) and asthma could help against Covid-19. In addition, in 2018, Novartis' NerveLive, declared that it was using information examination for mixing virtual and conventional preliminary models and decipher information precisely and all the more unequivocally which can prompt logical and clinical forward leaps.
Germany is prominent in Europe's virtual clinical trials market because of its strong healthcare infrastructure, favorable regulatory environment, and active pharmaceutical industry. Germany's commitment to digital-health innovations has meant researchers can engage in virtual clinical trials by being compliant with data protection laws under the General Data Protection Regulation (GDPR). Germany has various biotech clusters, such as Berlin and Munich that have partnered with universities for virtual studies. The government endorses various eHealth initiatives and funds digital clinical trials, which contributes to Germany's position at the helm of Europe's transition to decentralized forms of clinical research.
China is developing into the main player in Asia Pacific's virtual clinical trials market, aided by its fast-growing digital health ecosystem as well as reform-oriented government healthcare initiatives. The Chinese National Medical Products Administration (NMPA) is improving and accelerating its regulatory pathway, and as a result providers are now enabling innovative Remote trial technology. With some of the world's largest patient pools, a growing number of chronic diseases, and popular use of telemedicine platforms, it's not surprising China is conducive for virtual trials. In addition, collaborations with local CROs and global pharma to run decentralised studies in specific therapeutic areas, such as oncology and chronic diseases, is taking off in this emerging marketplace.
Market Overview
The virtual clinical trials market is growing significantly due to the cost-efficiency in clinical research work and flexibility. This market is filled with advanced tools which is used in collecting data and communicating with patients which allow to conduct trials which are efficient to draw results. This market includes several devices such as wearable devices, mobile health apps, and telemedicine. It enables to gathering of real-time data which further helps to take quick actions that can improve health conditions. This protects from major deterioration in the health condition of a patient having diseases such as coronary disease, high blood pressure, lung diseases, and many more.
Virtual Clinical Trials Market Growth Factors
The COVID-19 pandemic has altogether affected the market. The market is significantly determined by an ascent in R&D exercises, the rising medical services digitization, as well as reception of telehealth. In addition, mechanical progressions, unions between clinical exploration associations, drug, and biotechnology organizations as well as strong government drives are expected to drive the market. The pandemic of COVID-19 is making the clinical preliminary industry alter the approach to directing progressing or impending trials.
Nonetheless, more prominent measure of assortment information, demonstrate and oversee unwavering quality to the controllers, innovation disappointment, information exactness, are a portion of the difficulties that are expect to hamper the development of worldwide virtual clinical trials market. The development of the worldwide market is significantly determined as of late inferable from the Covid-19 episode across the globe. Biopharma organizations and administrative offices are holding back nothing innovations, virtual trials and telemedicine as they are the expected answers for restricting preliminary disturbances. The Food and medication Administration has proposed virtual visits and remote checking through telemedicine as an option in contrast to face to face visits. Thusly, organizations then again have pointed on drawing out a change in through mechanical improvement to meet quickly enlarging medical services digitalization. For example, in February 2020, Johnson and Johnson started a virtual preliminary plan Heartline study to looking at whether the Apple Watch and new iPhone application could diminish the chance of stroke finding atrial fibrillation.
Moreover, headway in innovation in the medical care industry are further expect to fuel the general development of the market. Direct delivery of IMPs (Investigational restorative items) to patients is the part of the decentralized (virtual) trials which has gotten forward movement throughout the course of recent years, and is supposed to upgrade the general market development across the globe.
Also, expanding events of individuals experiencing constant illnesses, irresistible sicknesses and neurological infections combined with rising geriatric populace are a portion of the variables driving the development of worldwide market, universally. For example, as per Center for Disease Control and Prevention, in the United States, on a normal 6 out of 10 individuals are experiencing a constant sickness and 4 out of 10 individuals are experiencing at least two, bringing about the main source of death in the country. This is additionally expected for fuel the mechanical improvement in the medical services industry and subsequently, expands the market development.
There has been an ascent in the R&D of new medications and immunizations that has expanded the volume and intricacy of clinical trials. Virtual clinical trials kill difficulties presented by conventional clinical trials for instance defers in quiet enrollment and tedious methods. Likewise, studies have uncovered that around 75.2% of individuals leaned toward a versatile preliminary more than conventional ones and 80.1% of patients are bound to take part in a clinical preliminary that utilizes portable innovation.
The market is recuperating at a critical speed after the pandemic as it offers different advantages to patients as well as patrons. During the pandemic, voyaging was possibly hazardous. It very well may be costly, in any event, bringing about lost compensation or expecting that childcare/eldercare be employed. These disadvantages limit introductory interest and give a straightforward answer for virtual clinical trials. Virtual clinical trials offer different advantages like successful information assortment, examination, and checking a lot of information progressively.
Virtual trials utilize observing gadgets, programming applications, online social commitment stages to direct every step of the clinical preliminary cycle including patient enrollment, guiding, estimating clinical endpoints, informed assent, and antagonistic responses. Telehealth, home consideration, and far off understanding checking has been picking up speed as a medical care offering, and the COVID-19 is adding more pull to this drive.
According to the continuum clinical report distributed in April 2020, roughly 30% of the overviewed clinical preliminary spots are projected to tremendously affect selecting patients for new preliminary examinations as well as holding as of now enlisted patients consistent with their review plans. Likewise, 81% of the European clinical preliminary review locales and 56% of the U.S. destinations showed that the patients are more averse to keep partaking in examinations. Furthermore, as of March 30, around 30 pharma or biotech organizations have detailed interruption to a preliminary because of the emergency. Virtual trials are otherwise called decentralized trials played a huge part to play in the COVID-19 emergency and are set to turn into a standard in the manner trials and certifiable examinations are run.
A virtual strategy allows individuals to participate in the preliminary from their homes guaranteeing exploration can proceed with in any event, when site visits can't, subsequently, addressing a clever methodology of gathering wellbeing and viability information from members of clinical examinations. Virtual visits and distant patient observing of face-to-face site visits give members a decision and genuine serenity of not being presented to pointless dangers. The virtual examinations empower supporters to remember a bigger populace for the review, hence further developing enlistment, commitment, and maintenance. Additionally, it empowers consistent constant information assortment through computerized wellbeing advances. In the end, virtual network, observing too as the executives can altogether diminish the work, time responsibility, and weight on the members, CRCs, and examiners.
Market Scope
| Report Coverage | Details |
| Market Size by 2024 | USD 10.72 Billion |
| Market Size by 2034 | USD 18.31 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 5.05% |
| North America Market Share in 2024 | 51% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Study Design, Indication, Phase, Geography |
Market Dynamics
Drivers
Rising Emphasis over Patient-Centric Approach
This market is highly focused on the patients' requirements which help the market to grow rapidly. It adds convenience to patients on tracking their health condition which attracts a huge number of patients towards using such devices. It reduces the need to travel from one place to another such development fuels the growth of the market. It helps patients living in any corner of the world and is considered highly travel-friendly. With the evolution of technology, it is becoming easy for patients to track their heart rate, blood pressure, oxygen level and many others that can be checked without any help from experts.
Opportunity
Technological Advancements
With the incorporation of various technologies in this market it raises its market players' opportunity to introduce a revolution in the market. The continuously growing number of patients suffering from chronic diseases and the rising healthcare sector in this field have enhanced the market potential drastically as it demands advancement in existing technology. It is highly beneficial for the remote patient as it becomes easy for them to track their health condition without any help. By the development of such technologies, it engages a wide number of patients and market players are working to fulfil their demand. As it is convenient to use it attracts a wide range of customers towards this market.
Study Design Insights
The interventional configuration portion drove the virtual clinical trials market in 2024. The quick expansion in the quantity of trials to foster novel drugs for different illnesses and the digitalization of research centers are factors driving the portion. The flare-up of Covid has raised the interest for testing and trials of new medications and immunizations to battle what is happening all over the planet as the customary strategy for clinical trials accompanies an enormous gamble of contamination in individuals. Consequently, moving the interest for interventional concentrate on plans.
The firm that initially begun this idea directed a whole interventional Phase2b "site-less" clinical preliminary with 372 patients across 10 states utilizing their restrictive versatile telemedicine-based stage to be specific "NORA". The extended admittance section is supposed to represent the most elevated CAGR over the estimate period. Extended admittance is a potential pathway that is proper where the possible advantage for the patient stifles the likely dangers, thus ceaseless development of new variations of COVID-19 is supposed to drive interest for extended admittance to new medications over a present moment.
Indication Insights
The oncology section was second largest revenue holder in 2024. The fragment is additionally expected to add to the most extreme portion of the market during the estimate time frame. This is ascribed to the rising instances of malignant growth all around the world and the rising number of oncology clinical trials. Disease patients are the most powerless during the COVID-19 pandemic. Agents and backers overseeing oncology clinical trials have in no time consolidated virtual and distant trials to keep patients safe and trials pushing ahead.
Also, disease scientists face extensive difficulties in persistent enrollment. As of June 2019, roughly 14, 000 oncology trials were effectively enrolling with a support pace of 3% to 8% of potential competitors, with a much more set number in minority and geriatric populaces. Low pace of enlistment presents dangers to the progress of explicit clinical examinations, they might hamper the treatment propels and comparing advantages to results. Thus, the low enlistment rate and the requirement for a different populace for oncology clinical examinations are expected to help the reception of virtual clinical trials.
- In October 2019, Georgetown University Medical Center involved cloud-based virtual interconnected figuring procedures for deducting the time in getting to malignant growth patients profile. Results showed that from 2014 to 2017, how much cases basically surveyed expanded from 46 to 622 when contrasted with the regular evaluations from 3 to 14 cases.
Virtual Clinical Trials Market Companies
- ICON, plc
- Parexel International Corporation
- IQVIA
- Covance
- PRA Health Sciences
- LEO Innovation Lab
- Medidata
- Oracle
- CRF Health
- Clinical Ink
- Medable, Inc.
Latest Announcements by Industry Leaders
- In June 2024, Bernd Haas, senior vice president, IQVIA Digital Products and Solutions expressed, “With site capacity becoming a real challenge for the industry, we believe it is our role and responsibility to deliver a solution that brings industry stakeholders together and relieves the burden on research sites. IQVIA One Home for Sites is the only product with a vision and scope broad enough to reverse the negative effects of technology proliferation that is plaguing sites today.”
Recent Developments
- In September 2024, PicnicHealth announced the launch a new program that lets researchers conduct clinical trials in cyberspace.
- In June 2024, IQVIA announced the launch One Home for Sites™, a new technology platform that acts as a single sign-on and a single dashboard for the key systems and tasks a clinical research site needs to perform across all of the clinical trials it is conducting.
Segments Covered in the Report
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Oncology
- Cardiovascular
- CNS
- Autoimmune/Inflammation
- Metabolic/Endocrinology
- Infectious Disease
- Genitourinary
- Ophthalmology
- Others
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Latin America
- Europe
- Asia-pacific
- Middle and East Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting