What is the Drug Repurposing Market Size?
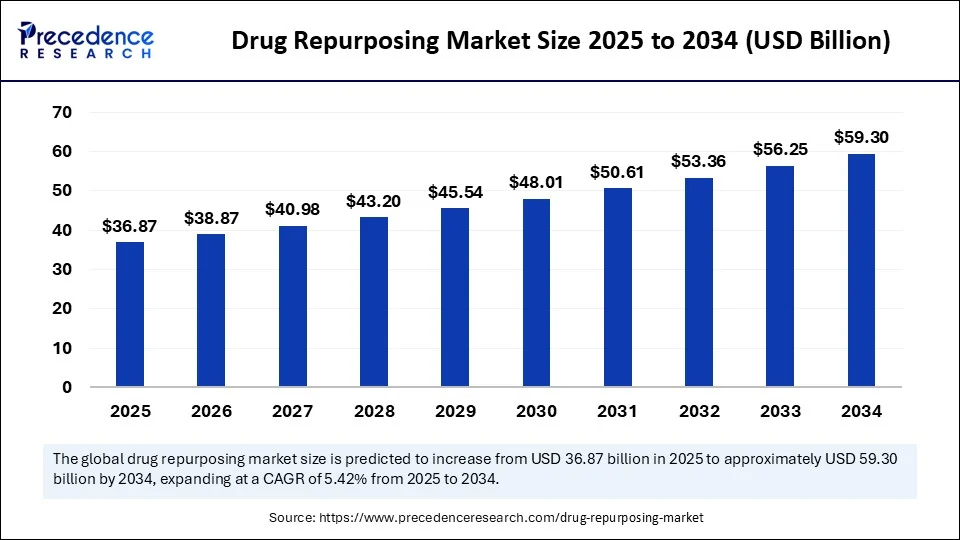
The global drug repurposing market size is accounted at USD 36.87 billion in 2025 and predicted to increase from USD 38.87 billion in 2026 to approximately USD 59.30 billion by 2034, expanding at a CAGR of 5.42% from 2025 to 2034. The growth of the market is attributed to the rising demand for cost-effective strategy to accelerate drug development by finding new uses for existing drugs, reducing risk, and enhancing patient outcomes.
Drug Repurposing MarketKey Takeaways
- In terms of revenue, the drug repurposing market was valued at USD 34.98 billion in 2024.
- It is projected to reach USD 59.30 billion by 2034.
- The market is expected to grow at a CAGR of 5.42% from 2025 to 2034.
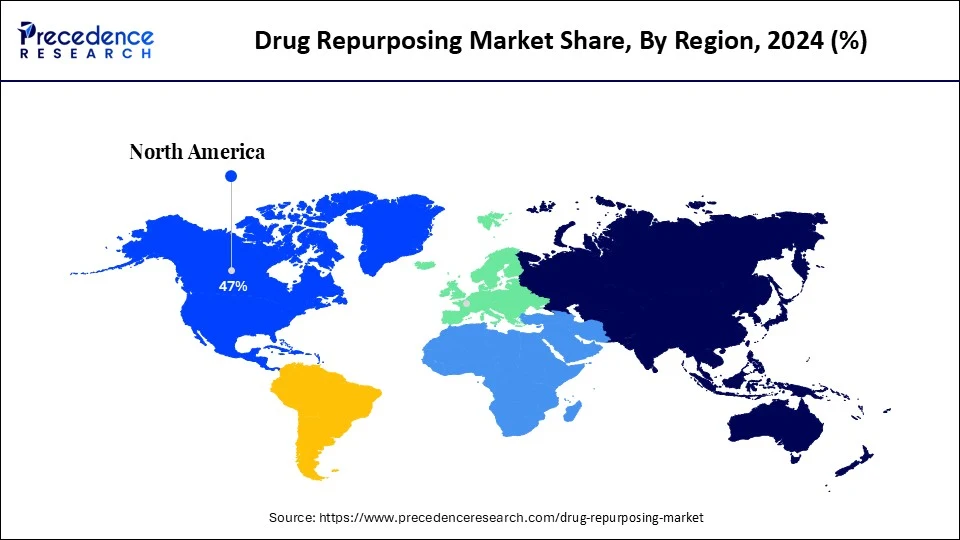
- North America held the largest revenue share of 47% in in 2024.
- Asia Pacific is anticipated to witness the fastest growth during the forecasted years.
- By type of approach, the disease-centric segment contributed the highest revenue share of 43% in 2024.
- By type of approach, the target-centric segment is expected to exhibit considerable growth in the market over the forecast period.
- By therapeutic area, the same therapeutic area segment held a significant revenue share of 68% in 2024.
- By therapeutic area, the different segments are anticipated to show considerable growth in the market over the forecast period.
- By drug molecules, the biologics segment accounted for the biggest revenue share of 62% in 2024.
- By drug molecules, the small molecule segment is anticipated to show considerable growth in the market over the forecast period.
Strategic Overview of the Global Drug Repurposing Industry
Drug repositioning, or repurposing, is a new approach to drug development. It involves finding new therapeutic uses for existing drugs, whether approved, discontinued, or still in clinical trials. Compared to traditional drug discovery, which is slow and costly, drug repurposing offers a more efficient and cost-effective path to market. This method's effectiveness relies on using existing clinical and pharmacological data, including off-label use, biological assays, and gene expression profiling, to speed up the drug development cycle.The drug repurposing market is experiencing significant growth due to the need for a rapid and cost-effective way to treat persistent diseases like cancer, Alzheimer's, and antibiotic-resistant infections. Research institutions and pharmaceutical companies are increasing their investment in this strategy to extend the lifespan of existing drugs and enhance their safety profiles.
Artificial Intelligence: The Next Growth Catalyst in Drug Repurposing
Artificial Intelligence is revolutionizing the drug repurposing market by accelerating the identification of new therapeutics, reducing development timelines and costs, and improving predictive accuracy through the integration of diverse data sources, leading to more personalized treatments. AI is revolutionizing drug repurposing by speeding up the identification of new treatments. This efficiency cuts down development time and costs, making repurposing more viable for both pharmaceutical companies and academic institutions. Furthermore, AI improves prediction accuracy by integrating data from sources like gene expression profiles, drug-target interactions, and patient records, allowing for more personalized drug repurposing for specific diseases or patient groups.
Market Outlook
- Market Growth Overview: The drug repurposing market is expected to grow significantly between 2025 and 2034, driven by the cost-effective and time-saving integration of AI, machine learning, and advanced bioinformatics platforms allows for more rapid and accurate identification of new therapeutic uses for existing drugs by analyzing vast datasets.
- Sustainability Trends:Sustainability trends involve resource efficiency and lean research & development, ethical and equitable access to medicines, and regulatory alignment with ESG goals.
- Major Investors:Major investors in the market include Novartis AG, Pfizer, AstraZeneca, Baillie Gifford and Platinum Falcon.
Startup Economy: The startup economy is focused on niche and orphan diseases, AI-driven discovery platforms, and efficiency and speed.
What are the Major Factors Boosting the Growth of the Drug Repurposing Market?
- Rising Prevalence of chronic diseases: Chronic diseases like cancer, Alzheimer's, and autoimmune disorders pose complex treatment challenges due to their increasing prevalence and intricate nature. These diseases often involve multiple genes and signaling pathways, making them suitable targets for drugs with polypharmacological properties. Repurposed pharmaceuticals with multiple targets offer a potential treatment solution.
- Innovations in bioinformatics and artificial intelligence: Computational biology, machine learning, and omics technologies have revolutionized drug repurposing. Tools like gene expression profiling, network pharmacology, and AI-guided modeling enable precise targeting of existing drugs to discover new indications.
- Quick approvals and regulatory support: Regulatory bodies like the FDA and EMA increasingly support drug repurposing through fast-track and orphan drug designations. This reduces development complexities and encourages companies to repurpose drugs, driving the drug repurposing market forward.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 59.30 Billion |
| Market Size in 2025 | USD 36.87 Billion |
| Market Size in 2026 | USD 38.87 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 5.42% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type of Approach, Therapeutic Area, Drug Molecules, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Growing Use of Repurposing Drugs for Cancer Treatment
The rising usage of repurposing drugs in cancer treatment is driving the growth of the drug repurposing market. Cancer management is notably complex and costly, marked by high failure rates in conventional drug development. Repurposing allows researchers to bypass early-stage trials, saving both time and resources, as the drugs' safety profiles are already established. Since the safety and efficacy profiles of repurposed drugs are often already known, the development timeline is considerably shortened. Drug repurposing also opens the doors for combination therapies with repurposed drugs to increase efficacy and inhibit drug resistance. The escalating application of drug repurposing in oncology efforts is stimulating market growth, also changing the paradigm of cancer therapy discovery and development, as cancer remains a major health burden to the global population.
Restraint
Intellectual Property Challenges
The complexity of intellectual property rights is one of the major restraining factors affecting the development of the drug repurposing market. Such legal and regulatory barriers make the process of innovation time-consuming and create challenges, particularly for small biotech companies. Furthermore, the absence of robust financial incentives associated with repurposed drugs is a challenge. Unlike novel drug discoveries, repurposed drugs often lack new patent protection, making them susceptible to generic competition. This non-exclusivity can discourage pharmaceutical firms from pursuing repurposing projects, as the return on investment may be considerably lower. Limited awareness among patients about repurposed drugs also hinder market growth.
Opportunity
Rising Investment in Orphan Drug Development
Repurposing drugs for rare diseases (orphan drugs) provides opportunities. Drug repurposing has become a key alternative for faster, cheaper drug development, particularly for unmet medical needs. Repurposed drugs, with their existing safety and pharmacokinetic data, allow for quicker clinical trials, making them financially attractive investments. This growing interest in collaborative investment and shared innovation will significantly improve drug repurposing, opening therapeutic innovation possibilities and solidifying its role in modern drug discovery.
Segment Insights
Type of Approach Insights
Why Did the Disease-Centric Segment Dominate the Drug Repurposing Market in 2024?
The disease-centric segment dominated the market and accounted for the largest revenue share of 43% in 2024, as it allows for a focused investigation of a specific disease, facilitating the identification of potential drug-disease relationships. This approach centers on a specific disease, investigating how existing drugs might apply based on their biological mechanisms. Researchers can potentially find drug-disease relationships more quickly by focusing on a disease. Furthermore, drug-disease connections can be found more quickly when the symptoms or disease pathways overlap with those of a known condition, because a given drug is already tested and approved in the case of an existing disease. Possession of safety and efficacy records of the original indication of the drug saves a lot of time and money since early-stage investigations can be avoided due to data availability.
The target-centric segment is expected to grow at a significant CAGR over the forecast period due to advancements in genomics, proteomics, and computational biology. This approach focuses on identifying known drug targets like proteins, enzymes, or receptors. It aims to understand how these targets are involved in various disorders beyond the drug's initial purpose. New technologies, including AI, machine learning, and network-based modeling, have significantly aided this process, helping researchers discover new target-disease connections. The target-centric approach is gaining importance with the rise of personalized medicine.
Therapeutic Area Insights
What Made the Same Therapeutic Area the Dominant Segment in the Market?
The same therapeutic area segment dominated the drug repurposing market with a major revenue share of 68% in 2024 because drugs are more likely to be effective if repurposed for similar conditions. Repurposing drugs within the same therapeutic area, like using a cancer drug for a different cancer, reduces uncertainty and risk because the drug has already shown some effectiveness. Diseases within the same therapeutic area share biological characteristics, making treatments effective across them. This method enables researchers to create new treatments more efficiently and provide solutions to patients who lack viable therapies.
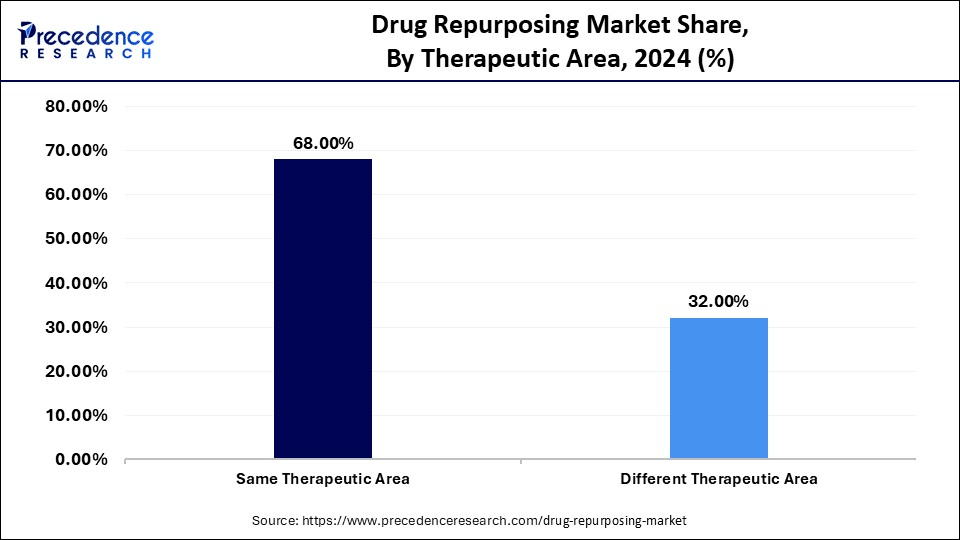
The different therapeutic area segment is expected to grow at a significant CAGR in the upcoming period. The growth of the segment is attributed to the rising demand for efficient and effective, highly cost-effective treatments across a wide variety of diseases, including oncology, neurological conditions, infectious diseases, and cardiovascular ailments. Drug repurposing helps pharmaceutical companies find new therapeutic applications for existing and even failed drugs, thereby shortening the development process in terms of time, cost, and the risks associated with new drug discovery. With the further development of computational biology, artificial intelligence, and data analytics, new indications are likely to be identified much faster, increasing the chances of successful outcomes.
Drug Molecules Insights
Why Did the Biologics Segment Dominate the Drug Repurposing Market in 2024?
The biologics segment led the drug repurposing market and accounted for the largest revenue share of 62% in 2024. This is mainly due to the increased demand for personalized medicine. Biologics, which include large, complex molecules like antibodies, proteins, and vaccines, are produced using living cells. A key advantage of biologics is their high specificity for biological targets, enabling more precise binding to disease-related molecules compared to small molecules. This precision can minimize side effects and enhance therapeutic effectiveness.Biologics are particularly useful for complex diseases such as cancer, autoimmune disorders, and chronic inflammation because of their targeted action. The growing demand for personalized and specific therapies is increasing the importance of biologics in drug repurposing. Advances in biotechnology and improved manufacturing processes are further facilitating their integration into medical practice.
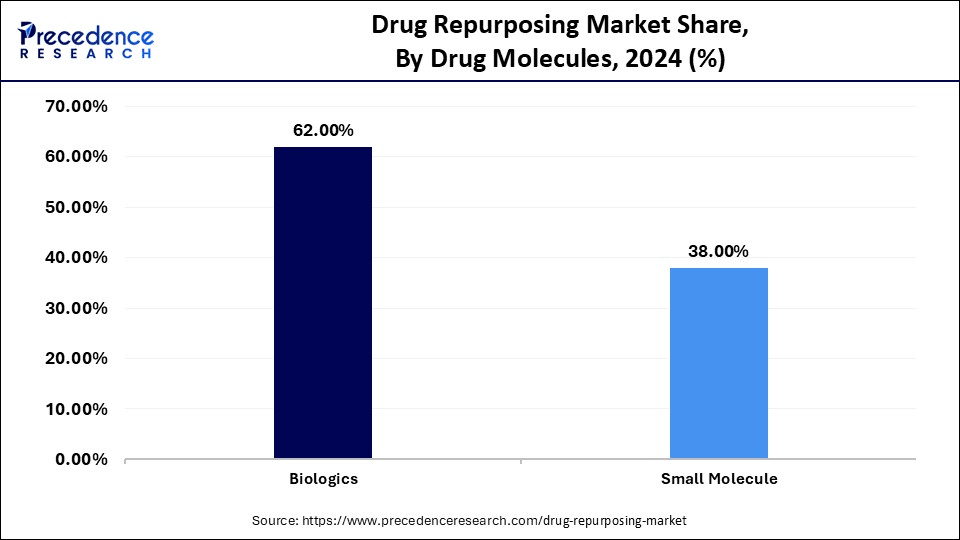
The small molecules segment is expected to grow at a significant CAGR over the forecast period, as repurposing small molecule drugs is often more cost-effective than developing new drugs from scratch. Small molecules refer to low molecular weight substances that may readily enter the cells and interact with the targets, which are usually enzymes or receptors, to modify the processes involved in the disease. They can be designed to maximize specific pharmacokinetics (absorption, distribution, metabolism, excretion), enabling oral administration and long-acting therapy. Their affordability and ease of production, compared to biologics, make them highly viable for repurposing. Furthermore, their ability to cross the blood-brain barrier makes them ideal candidates for neurological disorders like Alzheimer's and Parkinson's disease.
Regional Insights
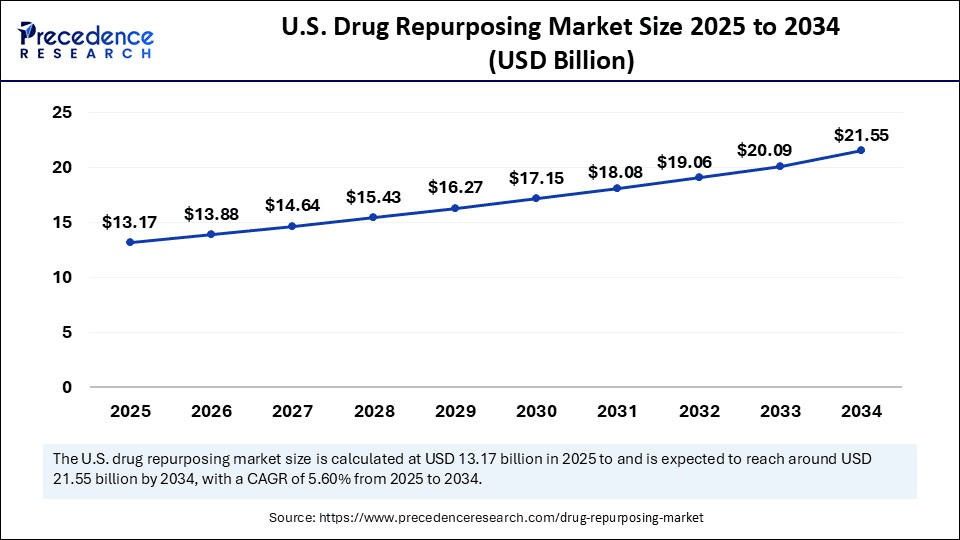
U.S. Drug Repurposing Market Size and Growth 2025 to 2034
The U.S. drug repurposing market size is exhibited at USD 13.17 billion in 2025 and is projected to be worth around USD 21.55 billion by 2034, growing at a CAGR of 5.60% from 2025 to 2034.
What Made North America the Dominant Region in the Market in 2024?
North America dominated the drug repurposing market with the largest revenue share of 47% in 2024. This is mainly due to the high prevalence of chronic conditions such as heart disease, cancer, and neurodegenerative diseases, creating an urgent need for new, cost-effective treatments. North America's advanced research infrastructure, along with supportive government policies and private sector investment, fuels the drug repurposing industry.
U.S. Drug Repurposing Trends
The U.S., in particular, benefits from government and private funding, including support from the National Institutes of Health (NIH) and other agencies. The presence of leading pharmaceutical firms and biotech start-ups in the U.S. further accelerates the development of innovative therapies through repurposing strategies.
Why is Asia Pacific Experiencing the Fastest Growth in the Drug Repurposing Market?
Asia Pacific is expected to grow at the fastest CAGR during the forecast period. Economic growth, increased healthcare spending, and rising prevalence of chronic and infectious diseases are driving the need for new, affordable treatments in this region. The use of modern technologies like artificial intelligence, bioinformatics, and big data analytics is accelerating drug development and discovery. Supportive government policies and increasing development of novel drugs are supporting the regional market growth.
China Drug Repurposing Market Trends
China is a major player in the market. This is mainly due to a surge in clinical trials focused on drug repurposing. Rising research and development activities and strategic alliances between local and foreign pharmaceutical companies further support market expansion. In addition, the increasing prevalence of chronic diseases creates the need for repurposing drugs for personalized medicine.
- In July 2024, NovaLead Pharma Private Limited (NovaLead), the pioneer of drug repurposing in India announced today that the drug regulator in India, CDSCO has approved their patented Repurposed Drug for treatment of Diabetic Foot Ulcer (DFU) which is a global unmet medical need. It is a new indication as well as a new formulation, and it is the first topical gel to be introduced in India that has been discovered and developed by NovaLead.
(Source:https://www.business-standard.com)
What are the Key Trends in the European Drug Repurposing Market?
The European drug repurposing market is expected to grow at a steady rate. This is mainly due to the increasing demand for repurposing drugs for new indications, especially in areas with unmet medical needs. The European Medicines Agency (EMA) plays a central role, offering incentives to encourage drug repurposing, such as orphan drug designation and adaptive licensing. Increased demand for personalized medicine and the incorporation of AI-driven drug discovery models are also boosting market growth in Europe.
U.K. Drug Repurposing Trends
The UK is emerging as the fastest-growing hub for drug repurposing. Its life sciences sector, with leading academic institutions and research centers, is driving innovation. Government projects and investment programs dedicated to drug repurposing have significantly boosted research efforts, supported by a well-establishedpharmaceutical industry and substantial investments in medical research.
Drug Repurposing Market Value Chain Analysis
Candidate Identification and Discovery
This initial stage involves identifying existing drugs or shelved compounds with potential for new therapeutic indications.
- Key Players: Recursion Pharmaceuticals, SOM Biotech, and Gero.
Preclinical and Clinical Development
Once a potential candidate is identified, it moves to preclinical evaluation using in vitro and in vivo models to assess its efficacy for the new indication.
- Key Players: Contract Research Organizations (CROs), Algernon Pharmaceuticals, and Academia and Research Institutes.
Regulatory Approval & Intellectual Property (IP)
This stage involves navigating the regulatory landscape, such as the FDA or EMA, to gain approval for the new indication.
- Key Players: Regulatory Agencies (FDA, EMA), Specialty Biotech and Pharma Companies, and Large Pharmaceutical Companies.
Manufacturing & Commercialization
The final stage involves scaling up manufacturing of the drug for the new indication and subsequent marketing, sales, and distribution to patients and healthcare providers.
Key Players: Pfizer, Novartis, Johnson & Johnson, and HLK Pharmacin.
Top Companies in the Drug Repurposing Market & Their Offerings
- Algernon Pharmaceuticals: This company exclusively focuses on drug repurposing, systematically screening a library of known drugs to identify new potential treatments for unmet medical needs.
- Biovista:Biovista utilizes a proprietary, AI-powered "Clinical Outcome Search Space" platform to uncover hidden relationships between drugs, diseases, and their underlying mechanisms. This data-driven approach allows for the efficient identification and validation of novel drug repurposing candidates.
- Celentyx Ltd:Celentyx contributes through specialized expertise in immunopharmacology and cell biology, performing experimental validation of drug repurposing hypotheses using human immune system models.
- ChemBio Discovery, Inc.: This company leverages advanced computational algorithms to screen chemical libraries against various disease targets, rapidly identifying potential new indications for existing molecules.
- Chord Therapeutics SA:Acquired by the Finnish company Faron Pharmaceuticals, Chord focused on developing repurposed drugs for specific inflammatory and rare diseases.
- Excelra: Excelra provides data-driven intelligence and analytics platforms that support the entire drug repurposing value chain, from initial hypothesis generation to clinical trial design.
- Fios Genomics: Fios Genomics offers bioinformatics and data analytics services that support drug repurposing by analyzing complex biological datasets to reveal new therapeutic links. They help clients identify novel uses for their existing drug portfolios.
- Lantern Pharma, Inc.: Lantern Pharma uses AI and machine learning to rescue and repurpose oncology drugs that previously failed clinical trials due to efficacy issues. Their platform, RADR, analyzes patient data to predict which patient populations might benefit from these rediscovered drug candidates.
- Novartis AG: As a major pharmaceutical giant, Novartis engages in drug repurposing primarily through internal R&D efforts and acquisitions, extending the lifecycle of its existing portfolio and exploring new therapeutic areas.
- Paradigm Biopharmaceuticals Ltd:This company focuses on repurposing pentosan polysulfate sodium (PPS) for the treatment of inflammatory diseases like osteoarthritis.
- Predictive Oncology: Predictive Oncology leverages AI and a biobank of tumor samples to predict which existing drugs, including off-patent ones, might be effective against specific cancer types.
- Segue Therapeutics, LLC:Segue Therapeutics focuses on developing treatments for rare and neglected diseases through its systematic drug repurposing platform. They work to bring viable, known-safety-profile drugs to market for conditions with high unmet needs.
- Sosei Group Corporation: This biopharmaceutical company engages in drug repurposing through its R&D efforts, often focusing on G-protein coupled receptors (GPCRs) and partnering with other companies to develop and commercialize novel therapeutics from existing compounds.
- Teva Pharmaceutical Industries: As a global leader in generic medicines, Teva plays a role in the commercialization of off-patent repurposed drugs, making treatments accessible at lower costs.
Recent Developments
- In June 2024, BioXcel Therapeutics used AI-based drug repurposing to find novel uses of drug candidates stalled in development. The company carries out therapeutic innovation at accelerated rates by integrating approved and established drugs, clinically validated candidates, big data, and the property of proprietary machine learning algorithms, and it is focused on research and development costs, market access, and is growth-oriented in neurology and immuno-oncology within the drug repurposing market.
- In October 2023, Rejuvenate Biomed ran a Phase 1b proof-of-concept clinical trial of RJx-01, a combination of galantamine and metformin in disuse-induced muscle atrophy. The trial was successful in all endpoints, indicating safety, tolerability, and good bioavailability. Since Phase 2 trials of sarcopenia are to be conducted in 2024, it underscores the problem of increasing participation in drug repurposing against age-related diseases.
- In August 2023, DEBRA UK declared that its first drug repurposing clinical trial had been approved, owing to funding by the A Life Free of Pain appeal. This project proposes to expedite the creation of treatment of Epidermolysis Bullosa (EB) by determining the potential new therapeutic applications of known drugs.
(Source: https://www.insideprecisionmedicine.com)
(Source: https://www.rejuvenatebiomed.com)
(Source: https://www.debra-international.org)
Segments Covered in the Report
By Type of Approach
- Disease-centric
- Target-centric
- Drug-centric
By Therapeutic Area
- Same therapeutic area
- Different therapeutic areas
By Drug Molecules
- Biologics
- Small molecule
By Region
- North America
- Asia-Pacific
- Europe
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting