Human PBMC Isolation Kit Market Size and Forecast 2025 to 2034
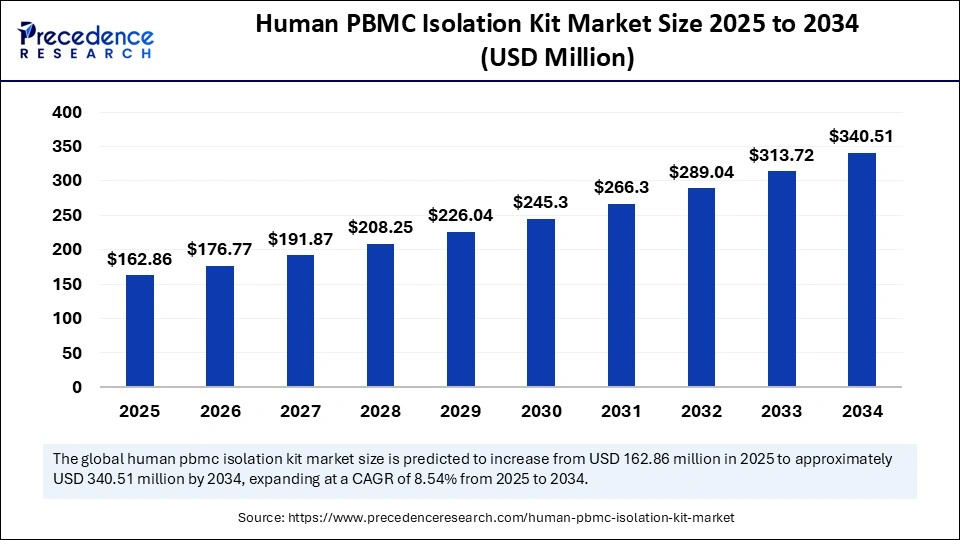
The global human PBMC isolation kit market size accounted for USD 150.05 million in 2024 and is predicted to increase from USD 162.86 million in 2025 to approximately USD 340.51 million by 2034, expanding at a CAGR of 8.54% from 2025 to 2034. The growth of the human PBMC isolation kit market is driven by rising investments in research and development for creating innovative therapies, ongoing clinical trials, and the expansion of the biotech industry.
Human PBMC Isolation Kit Market Key Takeaways
- The global human PBMC isolation kit market was valued at USD 150.05 million in 2024.
- It is projected to reach USD 340.51 million by 2034.
- The market is expected to grow at a CAGR of 8.54% from 2025 to 2034.
- North America dominated the global human PBMC isolation kit market with the highest share of 41% in 2024.
- Asia Pacific is expected to expand at the fastest CAGR in the market between 2025 and 2034.
- By product type, the density gradient medium segment captured the biggest market share in 2024.
- By product type, the cell separation columns segment is expected to grow at the fastest CAGR during the forecast period.
- By source of PBMCs, the whole blood segment contributed the highest market share in 2024.
- By source of PBMCs, the leukapheresis products segment is expected to show the fastest growth during the predicted timeframe.
- By application, the clinical research segment accounted for the major market share in 2024.
- By application, the pharmaceutical development segment is anticipated to witness the fastest growth over the forecast period.
- By end user, the academic and research institutes segment held the biggest market share in 2024.
- By end user, the biotechnology companies segment is predicted to grow at the fastest CAGR during the forecast period.
- By technology, the density gradient centrifugation segment accounted the biggest market share in 2024.
- By technology, the magnetic bead-based separation segment is anticipated to grow at a remarkable CAGR between 2025 and 2034.
Impact of Artificial Intelligence (AI) on the Human PBMC Isolation Kit Market
Integration of artificial intelligence in human PBMC isolation kits can help enhance the accuracy and efficiency of diagnostics and research applications. Automation of PBMC isolation processes can be applied for mitigating human errors, eliminating cross-contamination, enhancing sample processing throughput, and reducing hands-on time by using AI-powered robotic systems, such as RoboSep-S, developed by Stemcell Technologies offers automation of immunomagnetic PBMC separation.
Furthermore, AI can be used for various data analysis and interpretation applications in big data analytics for studying large datasets available from PBMC experiments for predicting disease occurrence and imitating pharmacological action, in image analysis for analyzing the function and structures of PBMC cells as well as in gene expression analysis for identifying relevant biomarkers and developing predictive models for disease diagnosis and stratification.
U.S. Human PBMC Isolation Kit Market Size and Growth 2025 to 2034
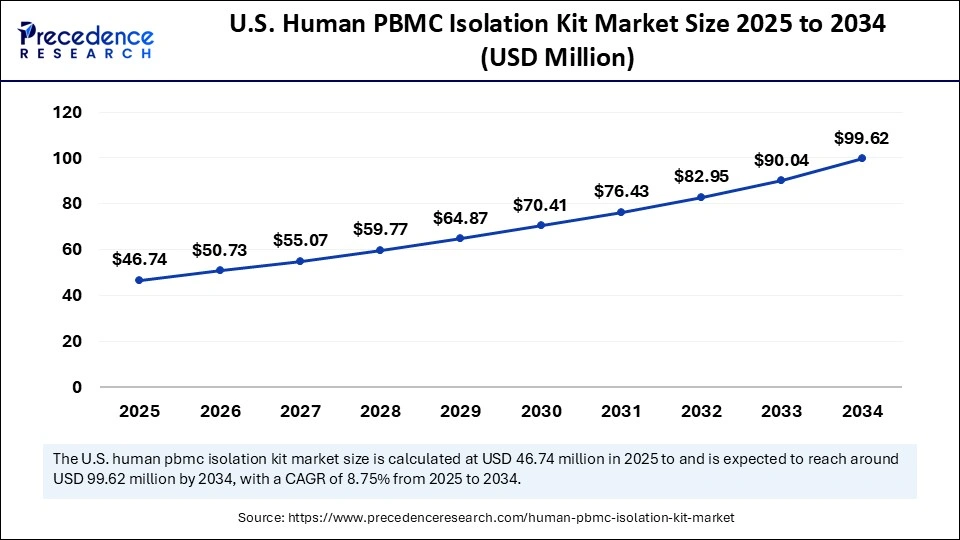
The U.S. human PBMC isolation kit market size was exhibited at USD 43.06 million in 2024 and is projected to be worth around USD 99.62 million by 2034, growing at a CAGR of 8.75% from 2025 to 2034.
North America registered dominance in the global human PBMC isolation kit market by capturing the largest share in 2024. The region's dominance is attributed to increased chronic and infectious disease burden and the presence of advanced healthcare infrastructure. A significant number of universities and research institutions in the region are focusing on research in hematology, immunology, and other relevant fields, creating the need for human PBMC isolation kits, which are crucial for developing innovative therapies and studying the immune system.
The U.S. is a major player in the human PBMC isolation kit market within North America. The growing awareness and demand for personalized treatments, rising investments by government bodies and private investors fostering advancements in research, increased focus on developing effective, innovative therapies, and surging approvals from the U.S. FDA are driving the market expansion.
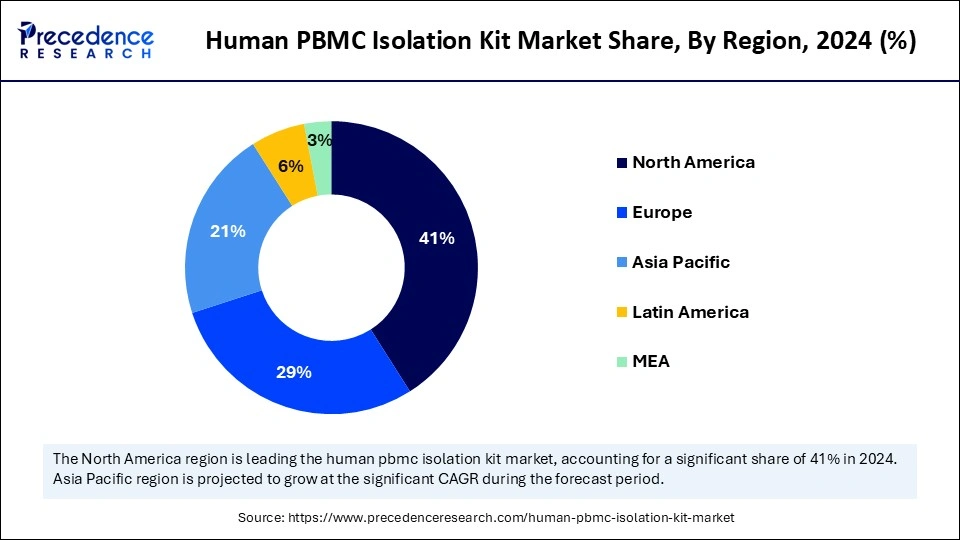
Asia Pacific is expected to grow at the highest CAGR during the forecast period. The market growth in the region is driven by increased healthcare expenditure, adoption of advanced diagnostics and treatment technologies, expanding biopharmaceutical sectors, growing presence of Clinical Research Organizations (CROs), and rising research collaborations between academic institutes, pharmaceutical companies, and government bodies. Key regional players like China, Japan, and India are driving the market expansion with increased investments in research and development activities.
China is expected to grow at the fastest rate in the human PBMC isolation kit market within Asia Pacific due to the rising investments in the healthcare sector, advancements in the biopharmaceutical industry, and surging research collaborations. With the increasing prevalence of various life-threatening diseases, there is a high demand for personalized therapies, contributing to market growth.
Europe is expected to grow at a notable rate over the forecast period. The region's market growth can be linked to the presence of strong research infrastructure, rising focus on personalized medicine for tailoring patient-specific treatments, expansion of manufacturing facilities, and supportive regulatory frameworks. Germany, the UK, and France are significant market players fostering the region's growth. Germany is expected to grow at a significant rate in the market over the forecast period. The continuous developments in the biotechnology sector, increased research expenditures, and strong healthcare systems are fuelling the market growth.
Market Overview
A human PBMC isolation kit refers to a tool utilized in clinical and research settings for isolating peripheral blood mononuclear cells (PBMCs) from whole blood or other blood products, making them available for several applications, such as functional studies, flow cytometry, and cell culture. Human PBMCs comprise B cells, T cells, NK (natural killer) cells, lymphocytes, monocytes, and dendritic cells.
The rising healthcare expenditure around the world is boosting the demand for diagnostic tests for several infectious diseases and for monitoring immune responses after organ transplants. Government initiatives and funding fostering innovations in biomedical research, expansion of PBMC manufacturing facilities and service providers, and growing emphasis on developing sophisticated isolation kits with multiple features are the factors fostering the growth of the human PBMC isolation kit market.
Human PBMC Isolation Kit MarketGrowth Factors
- Demand for personalized medicine: PBMCs are essential in research applications for understanding underlying mechanisms and treatment responses of individual patients. The ongoing advancements in isolation of specific cell types and subtypes from patient samples are further driving the focus on developing personalized treatment strategies.
- Increased emphasis on R&D activities: The rising prevalence of chronic diseases like cancer and autoimmune disorders creates the need for better comprehension of immune responses. Additionally, rising investments are fostering the research and development processes in immunology and oncology for developing innovative therapies.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 340.51 Million |
| Market Size in 2025 | USD 162.86 Million |
| Market Size in 2024 | USD 150.05 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.54% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Deployoment, Technology, End User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Expanding Scope of Applications
Human peripheral blood mononuclear cells are widely being used in different clinical and research applications, which is driving the demand for isolation kits. PBMCs find applications in diagnostics as well as therapeutics. The growing number of studies in oncology, infectious diseases, and regenerative medicine further drive market's growth. To meet the globally surging demand, manufacturers are highly focused on developing automated systems and closed-system technologies, implementing microfluidics-based approaches, optimizing reagent formulations, and using automated controlled-rate freezers. Furthermore, increased emphasis on understanding customer needs, identifying unmet needs, and developing tailored products and services while maintaining compliance with regulatory standards are also driving the market growth.
Restraint
High Costs and Ethical Concerns
Several researchers, small-scale laboratories, and biotech companies on a shoestring budget are not able to accept highly advanced and expensive PBMC isolation kits, which further restrain the market growth. Furthermore, the issues and ethical concerns related to acquiring human blood samples for clinical research and commercial use while following statutory obligations like maintaining the privacy of data and informed consent can make it difficult for biotechnology businesses and research laboratories to obtain blood samples. The quality of PBMCs varies depending on the blood sample's sources, affecting the reliability of the isolation process.
Opportunities
Advancements in Precision Diagnostics and Immunology
Improvements in human PBMC isolation kits, like enhanced purity, reduced cell stress, and improved cell viability, have been achieved with the development of protocols for using specific media and gentle handling. Implementation of multi-omics approaches such as genomics and proteomics for better understanding of PBMC function, use of single-cell technologies for identifying rare cell populations, 3D cell culture models like organoids, and development of multiplexed assays such as ELISpot and FluoroSpot assays for comprehensive immune profiling are enabling the development of efficient and high-yield PBMC isolation kits. In addition, the increasing number of clinical research for developing regenerative medicine creates lucrative growth opportunities in the market.
Product Type Insights
The density gradient medium segment dominated the human PBMC isolation kit market with the largest share in 2024. Density gradient centrifugation is the most widely used method for human PBMC isolation and requires density gradient media like Ficoll-Paque and Lymphoprep, which are used extensively due to their cost-effectiveness and efficiency for PBMC separation. The increased focus of manufacturers on implementing go-to-market strategies such as targeting primary audiences like researchers in virology and immunology, among others, as well as secondary audiences including clinical laboratories and hospitals, developing density gradient media of low-cost and multiple applications with the ability for isolating PBMCs with high purity and yield further ensuring reliability of results in downstream applications, use of online and offline marketing channels like social media and scientific conferences among others are the factors driving the growth of this segment.
The cell separation columns segment is expected to grow at the fastest rate during the forecast period. The growth of this segment can be attributed to the rising demand for cell therapies in research applications, advancements in techniques like immunomagnetic cell separation (column-based or column-free) used for high-purity and high-yield PBMC isolation, as well as increased investments by manufacturers for developing innovative technologies to expand their product portfolios.
Source of PBMCs Insights
The whole blood segment dominated the human PBMC isolation kit market with a major share in 2024. Human PBMC isolation kits are designed specifically to work directly with whole blood samples as an input for simpler and more efficient isolation processes while ensuring cell viability for downstream applications. The increased use of human PBMCs in various clinical and research applications, such as studying cytokine expression, signal transduction, inflammatory responses, immune monitoring, and the evaluation of the efficacy of immunotherapies, bolstered segmental growth.
The leukapheresis products segment is expected to grow at the fastest rate during the predicted timeframe. Leukapheresis products or leukopaks are enriched with mononuclear leukocytes (monocytes and lymphocytes) in comparison to whole blood, providing a high concentration of PBMCs with increased yields and streamlined downstream processing. These products support chemical-free processing, scalable isolation, and also help in maintaining the consistency and reproducibility of experimental results by minimizing donor-to-donor variability and reducing contamination, further making them valuable for different applications such as the development of immunotherapies, immunology studies, and in cell therapy research.
Application Insights
The clinical research segment dominated the human PBMC isolation kit market by holding the maximum share in 2024. This is mainly due to the increased clinical studies in areas like drug development, cancer, and cell therapies. The rising research on personalized medicine for addressing individual variations in immune responses, in the diagnosis of autoimmune diseases, for studying infectious diseases, and for developing safe and effective vaccines further bolsters segmental growth. Furthermore, ongoing advancements in creating innovative research techniques and applications for PBMCs, demand for systematic and standardized isolation methods, rise in the number of facilities and laboratories compliant with Good Clinical Laboratory Practices (GCLP), huge number of ongoing clinical trials and initiatives by various governments for funding immunology and biomedical research projects are the factors driving the market growth of this segment.
The pharmaceutical development segment is expected to grow at the fastest rate over the forecast period. Technological advancements in pharmaceuticals, implementation of buoyancy activated cell sorting (BACS) technique for improved cell separation with microbubble technology, cell preparation tubes allowing on-site processing in time-critical clinical trial protocols, and automation of PBMC isolation systems are reducing cross-contamination risks and increasing sample throughput. Additionally, the increased use of human PBMC isolation kits for immune monitoring of antigen-specific T cells, development of innovative platforms and technologies by various biopharmaceutical companies as well as the implementation of these kits in various pharmaceutical applications such as for biomarker identification, patient stratification, and for research in immune-oncology, toxicology and rare disease are the factors expected to drive the market growth of this segment in the upcoming years.
End User Insights
The academic & research institutes segment dominated the market with the largest share in 2024. This is mainly due to the increased focus of researchers on developing effective immunotherapies and cell-based therapies. The rising collaborations between research institutes and biotech companies, growing adoption of advanced and automated technologies, and various government initiatives for funding biomedical research projects are fostering market growth. Furthermore, advancements in cryopreservation technologies enabling longitudinal studies, the need for developing standardized procedures and harmonization of quality control measures for ensuring result consistency and mitigating variability in research, as well as academic institutions' contribution to developing a skilled and knowledgeable workforce, are fuelling the market expansion.
The biotechnology companies segment is expected to grow at the fastest rate during the forecast period. Biotechnology companies are focused on adopting simpler and streamlined methods such as automated systems for more reproducible and faster isolation of PBMCs, microfluidics-based approaches for reducing reagent consumption and higher throughput, use of immunomagnetic negative selection for eliminating unwanted cells (RBCs, granulocytes and platelets) resulting in improved purity and recovery with minimal cell handling and reduced costs. Moreover, increased use of PBMC cells for downstream applications, such as in flow cytometry and DNA/RNA extraction, among others, growing demand for personalized medicine, and rising merger and acquisition activities are driving the market growth of this segment.
Technology Insights
The density gradient centrifugation segment dominated the human PBMC isolation kit market with the largest share in 2024. The density gradient centrifugation (DGC) technique is applied to separate blood components on the basis of their density by using a density gradient medium. Ongoing technological advancements, such as the development of microfluidic devices and miniaturized systems for faster and high-throughput separations in large-scale applications, optimizing gradient media and techniques for enhancing cell viability and separation, and robotic liquid handling workstations for automating the PBMC isolation process, leading to more reproducible and standardized outcomes, are driving the market growth of this segment.
The magnetic bead-based separation segment is expected to grow at the fastest rate during the forecast period. Magnetic bead-based separation techniques, such as magnetic-activated cell sorting (MACS), column-free MACS, and Dynabeads, are known for their superparamagnetism and monodispersity and are being extensively used for human PBMC isolation. Furthermore, the increased emphasis of researchers on developing innovative bead materials with optimized surface chemistry, such as graphene-coated beads and hydrogel-based beads, growing use for isolation of rare cell populations like in circulating tumor cells (CTCs) for enhancing cancer research and diagnostics, evaluation of downstream assay compatibilities with different magnetic bead types in fluorescent immunohistochemistry and nucleic acid extraction as well as focus on safeguarding cell viability and functionality while reducing cell stress in the separation process are the factors fuelling the market expansion.
Human PBMC Isolation Kit Market Companies

- Abcam
- Amsbio
- BioVision
- Miltenyi Biotec
- Stemcell Technologies
- Thermo Fisher Scientific
- Fabgennix International Inc
- Zen-Bio
Recent Developments
- In March 2025, Honourable Anita Anand, Canada's Minister of Innovation, Science and Industry, declared an investment of $49.9 million in STEMCELL Technologies Canada Inc. through the Strategic Innovation Fund. The funding supports the company's $222 million project for building two new state-of-the-art biomanufacturing facilities which will facilitate the large-scale production of critical inputs utilized in the development and manufacturing of therapeutics, vaccines and diagnostic technologies as well as advancements in other innovative technologies such as immunotherapy, tissue engineering, and cell and gene therapies.
- In May 2023, Akadeum Life Sciences announced the launch of a new series of products for cell therapy research and development, enabling direct leukopak human immune cell isolation and T-cell activation/expansion kits at the American Society of Gene + Cell Therapy's 26th Annual Meeting in Los Angeles, California.
Segments Covered in the Report
By Product Type
- Cell Separation Columns
- Density Gradient Medium
- Separation Kits (Magnetic, Non-magnetic)
- Filtration Devices
By Source of PBMCs
- Buffy Coat
- Bone Marrow
- Whole Blood
- Leukapheresis Products
By Application
- Diagnostics
- Clinical Research
- Pharmaceutical Development
- Stem Cell Research
- Immunology Studies
By End User
- Academic & Research Institutes
- Biotechnology Companies
- Clinical Laboratories
- Pharmaceutical Companies
By Technology
- Affinity Chromatography
- Density Gradient Centrifugation
- Filtration Techniques
- Magnetic bead-based Separation
By Region
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- Latin America
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting