What is Inhaled Nitric Oxide Delivery Systems Market Size?
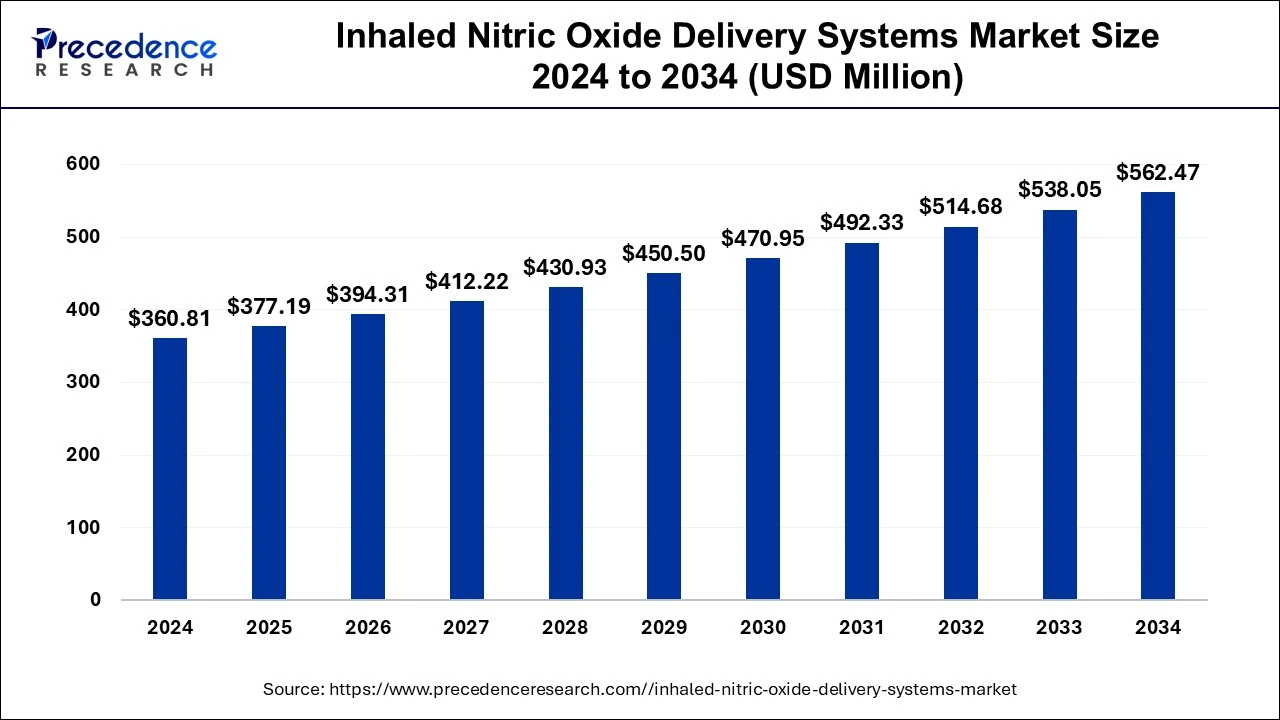
The global inhaled nitric oxide delivery systems market size accounted for USD 377.19 million in 2025 and is expected to exceed around USD 562.47 million by 2034, growing at a CAGR of 4.54% from 2025 to 2034. The rising demand for rapid drug delivery devices with the ease in use and convenient for the patient with any types of respiratory disease are contributed in the growth of the market.
Market Highlights
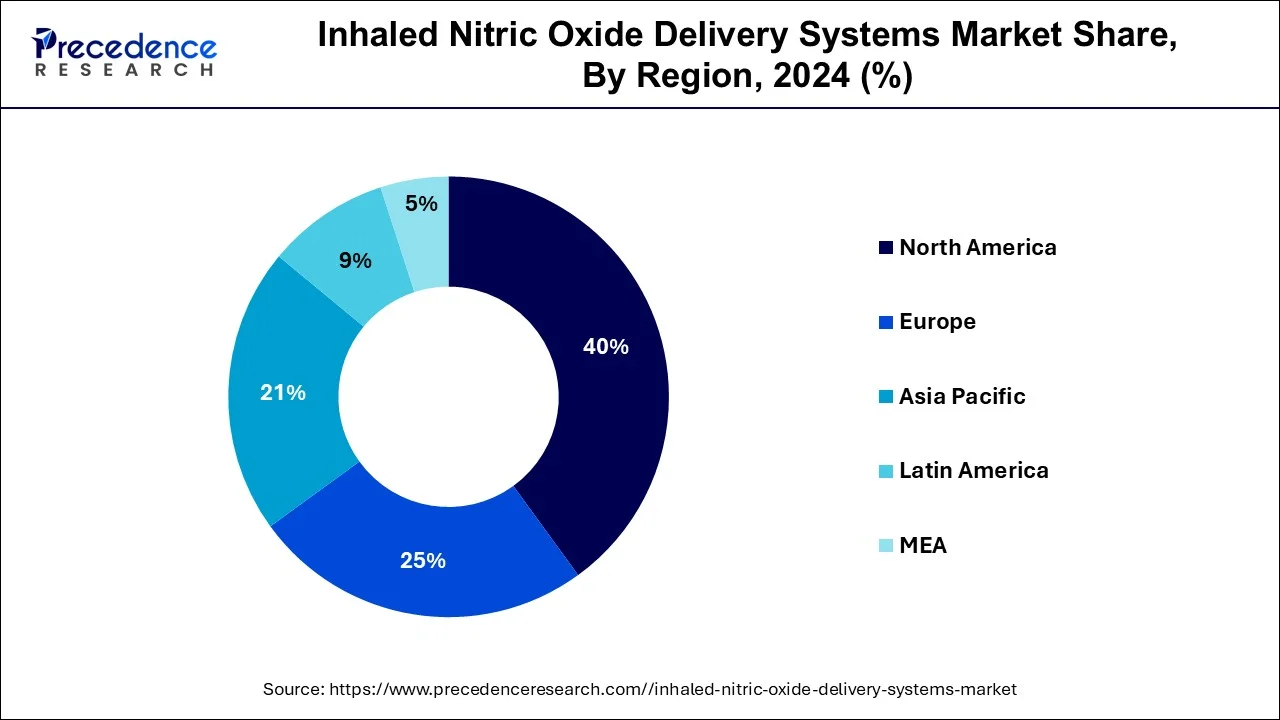
- North America dominated the global market with the largest market share of 40% in 2024.
- Asia Pacific is expected to show significant growth during the forecast period.
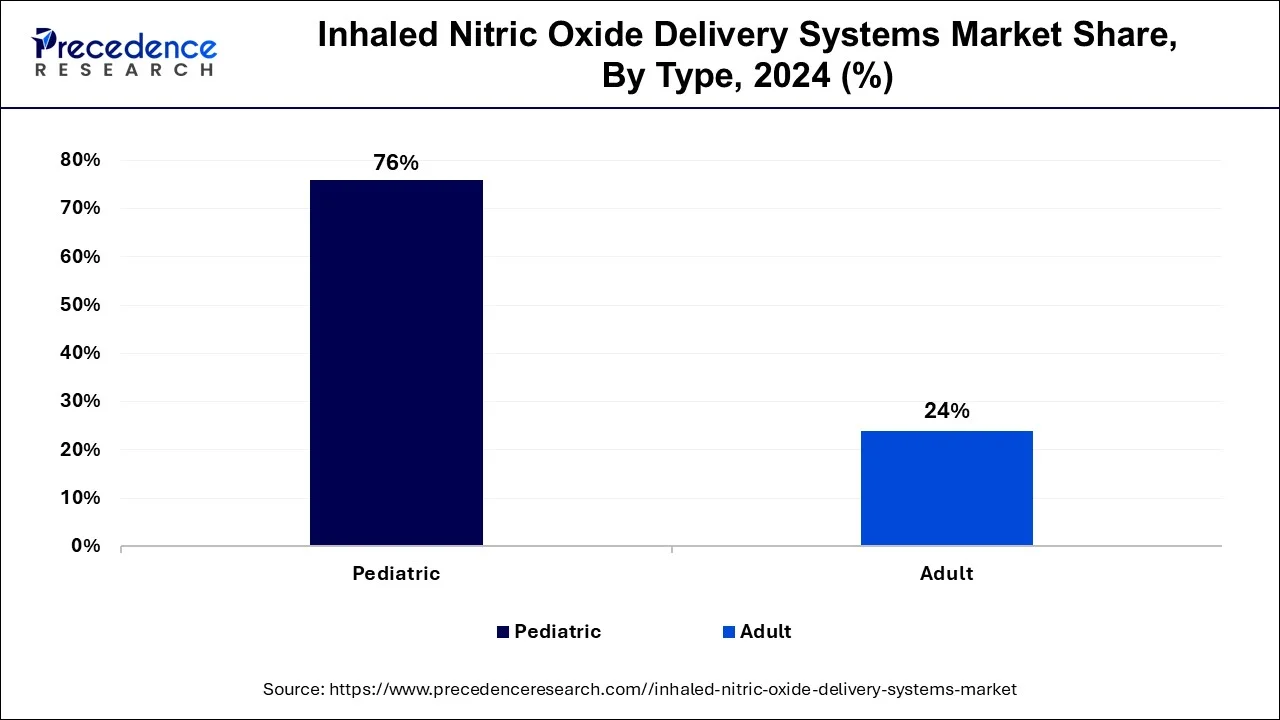
- By type, the pediatric segment contributed the highest market share of 76% in 2024.
- By type, the adult segment will witness significant growth during the forecast period.
- By product, the disposable segment captured the biggest market share in 2024.
- By product, the system segment will show rapid growth during the forecast period.
- By application, the hypoxic respiratory failure (HRF) segment generated the major market share in 2024.
- By application, the adult respiratory distress syndrome (ARDS) segment is expected to grow at the fastest CAGR during the forecast period.
- By end-user, the hospital segment has held the largest market share of 81% in 2024.
- By end-user, the ambulatory centers segment is projected to grow at the fastest CAGR in the future years.
What are inhaled nitric oxide delivery systems?
The inhaled nitric oxide delivery system is the medication process in which the nitric oxide is inhaled through the mouth and nose. It is the efficient delivery system that helps in effectively delivering the nitric oxide drug in a certain proportion into the body by the mouth or the nose. The inhaled nitric oxide delivery system is only given by the physician. There is an increasing prevalence of chronic respiratory disease in the population.
How Can AI Impact the Inhaled Nitric Oxide Delivery Systems Market?
The adaptation of artificial intelligence into respiratory disease treatment enhances patient care with continuous monitoring of patient data such as blood gas levels, respiratory patterns, and lung mechanics. AI provides real-time insights into the ventilation parameters that ensure optimal support for patients' respiratory systems. The AI also enhances the quality of personalized respiratory treatment by detecting and analyzing patient history, symptoms of the condition, and response to therapies. AI-based algorithms can detect and analyze the symptoms of respiratory disease early and help physicians make informed decisions for treatment.
- In November 2024, SoundHealth, Inc., a healthcare technology organization focusing on the power of artificial intelligence and medical science to enhance patient outcomes, launched the SONUCheck, the innovative vocal analysis feature in the SONU app that uses voice noninvasive biomarker for measuring the nasal patency.
Inhaled Nitric Oxide Delivery Systems Market Growth Factors
- Increasing penetration of COPD: The rising number of cases of chronic obstructive pulmonary disorders in the population due to several factors is driving the demand for an effective and faster medication delivery system.
- Increasing healthcare infrastructure: The growing healthcare infrastructure and the development of medication for rapid drug delivery, as well as the ongoing investment in pharmaceuticals and biomedical applications.
- Government interventions: The increasing government intervention in the development of favorable initiatives and policies in the development of the healthcare infrastructure and the launch of new medication and delivery systems are accelerating the growth of the market.
Market Outlook
- Industry Growth Overview: The inhaled nitric oxide delivery systems market is increasing, driven by the rising prevalence of respiratory and cardiovascular diseases such as COPD and neonatal hypoxic respiratory failure. Development in technology, including more portable and digitally monitored schemes, and expanding clinical research in novel applications such as neuroprotection.
- Global Expansion: The inhaled nitric oxide delivery systems market is increasing worldwide, driven by the growing prevalence of respiratory diseases among the pediatric and adult populations, like neonatal hypoxic respiratory failure and COPD. North America is dominated in the market as innovations in delivery systems, like portable and tankless tools, such as Vero Biotech's Genosyl DS.
- Major investors: Major investors in the inhaled nitric oxide delivery systems industry include large pharmaceutical and medical device companies such as Mallinckrodt Pharmaceuticals, Getinge AB, and Linde plc, as well as specialized biotech organizations such as Vero Biotech and Beyond Air, Inc.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 377.19 Million |
| Market Size in 2026 | USD 394.31 Million |
| Market Size by 2034 | USD 562.47 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 4.54% |
| Leading Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Product, Application, End-user, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
The increasing awareness regarding nitric oxide
The rising use of nitric oxide in the healthcare industry for the treatment of several diseases and other awareness regarding the benefits associated with nitric oxide usage is accelerating the adoption of inhaled nitric oxide delivery systems. Nitric oxide supplements are used in different disease treatments, such as reducing high blood pressure, maintaining the blood flow in the body, treating erectile dysfunction, reducing muscle soreness, lowering blood pressure, and others.
Restraint
Side effects
The side effects associated with nitric oxide, such as its rapid reaction with the oxygen in the lung and form the nitrogen dioxide, potentially develop the pulmonary irritant, which may cause severe issues in the body that limit the adoption of the inhaled nitric oxide delivery systems market.
Opportunity
Incorporation of smart technology
The integration of smart technologies into the inhaled nitric oxide delivery systems, such as the incorporation of alarms and automatic shut-off features. The integration of smart features includes higher precision and control, real-time monitoring, enhanced safety features, and versatility. The government and industry leaders are increasing investments in the development and launch of new products.
Segment Insights
Type Insights
The pediatric segment dominated the inhaled nitric oxide delivery systems market in 2024. The increasing penetration of respiratory disease in children due to genetics, environmental pollution, and other factors that cause the increased number of diseases, such as Persistent Pulmonary Hypertension and Hypoxic Respiratory Failure (HRF) of Newborns (PPHN), boosts the demand for inhaled nitric oxide delivery systems. Children and infants with pulmonary hypertension are treated with inhaled nitride oxide as it improves the ventilation-perfusion mismatch and reduces pulmonary vascular resistance. Continuous research and development programs contribute to further launching medication products.
- Asthma patients recorded 94,000 hospital inpatient stays and more than 900,000 visits to the Emergency department. There are 7.7% of Americans who have asthma, 24.9 million of the population have asthma, 20.2 million adults, and 4.6 million children. 147,382 deaths occurred due to chronic lower respiratory disease, including asthma, in 2022.
The adult segment will witness significant growth in the inhaled nitric oxide delivery systems market during the forecast period. The rising prevalence of chronic respiratory illness in the adult population, such as COPD, Acute Respiratory Distress Syndrome (ARDS), and others, is driving the growth of the inhaled nitride oxide delivery systems market. The rising awareness about nitric oxide supplements enhances performance during exercise, increases heart health, enhances the healing process, reduces and maintains high blood pressure, erectile dysfunction, and others.
Product Insights
The disposable segment accounted for a significant share of the inhaled nitric oxide delivery systems market in 2024. The increasing demand for single-use disposable medication products is driving the demand for the disposable inhaled nitric oxide delivery system. The increasing awareness regarding infectious diseases and cross-contamination is driving the demand for disposable medication products or systems.
The system segment will show rapid growth in the inhaled nitric oxide delivery systems market during the forecast period. The increasing adoption of the modern system due to ease of use and the increasing demand for the modernization of the medication system or devices from different healthcare settings, including clinics, hospitals, ambulatory services, and others.
Application Insights
The hypoxic respiratory failure (HRF) segment led the global inhaled nitric oxide delivery systems market in 2024. There is an increasing demand for nitric oxide supplements for the treatment of hypoxic respiratory failure owing to their medication benefits, such as improving ventilation-perfusion mismatch and reducing pulmonary vascular. The rising penetration of hypoxic respiratory failure in pediatric patients, such as infants and children.
The adult respiratory distress syndrome (ARDS) segment will observe substantial growth in the inhaled nitric oxide delivery systems market during the predicted period. The increasing number of respiratory distress syndrome in adults due to changing lifestyles, increasing adoption of habits like smoking, and environmental pollution are collectively driving the demand for nitric oxide supplements for the treatment of the disorders.
End-user Insights
The hospital segment accounted for the largest share of the inhaled nitric oxide delivery systems market in 2024. The higher number of patients visiting for their health checkups and for the treatment of several diseases is causing the increasing demand for nitric oxide supplements by hospitals. The hospital is one of the leading healthcare institutes, has a larger capacity for patient administration, and is highly equipped with modern healthcare materials and equipment for the efficient treatment process.
The ambulatory centers segment will show significant growth in the inhaled nitric oxide delivery systems market during the forecast period. The rising popularity of ambulatory healthcare centers is due to the availability of all the essential and important equipment that is important for the treatment of patients. The ambulatory centers are cost-effective solutions to the expensive healthcare solutions.
Regional Insights
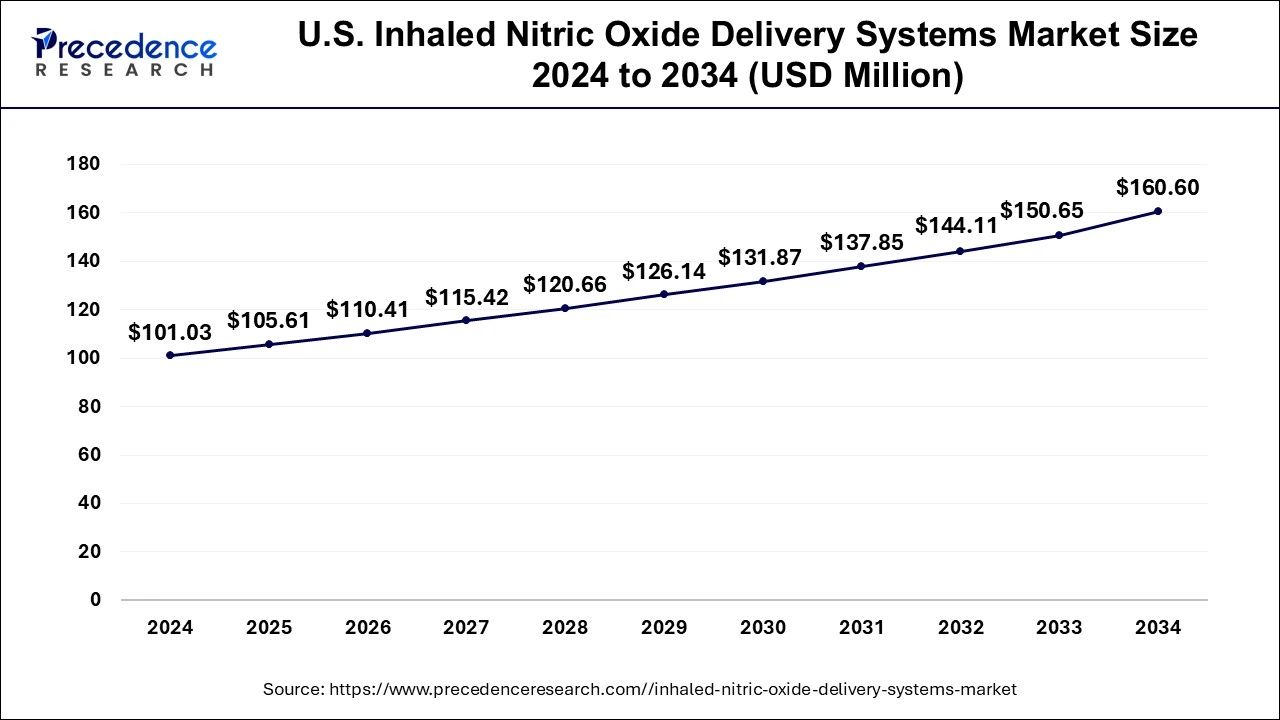
U.S. Inhaled Nitric Oxide Delivery Systems Market Size and Growth 2025 to 2034
The U.S. inhaled nitric oxide delivery systems market size is exhibited at USD 105.61 million in 2025 and is projected to be worth around USD 160.60 million by 2034, growing at a CAGR of 4.74% from 2025 to 2034.
U.S.: Strong R&D and innovation
The U.S. has the growing healthcare spending in the world, which drives the adoption of costly, new treatments such as iNO. The country has a well-established medical care infrastructure with hospitals and clinics equipped to administer iNO therapy. Important R&D is conducted in the U.S., leading to novelties in both the delivery systems and the increasing use of iNO's therapeutic applications.
North America: Technological advancement in the pharmaceutical industry
North America dominated the inhaled nitric oxide delivery systems market in 2024. The growth of the market is attributed to the rising infrastructural development and the continuous investment by the regional government in the development of the healthcare system. The continuous advancement in the healthcare technology and pharmaceutical industry is driving the growth of the market. The increasing prevalence of respiratory disease in the population due to the changing environmental pollution, genetics, and changing lifestyles, such as the rising adoption of smoking, drives the demand for the efficient treatment of respiratory disease.
- In the United States, there are 4.3% of adults diagnosed with COPD, emphysema, or chronic bronchitis in 2023. 4.2% of COPD patients visit office-based physicians. 791,000 visits take place in emergency departments with COPD as the primary diagnosis.
Asia Pacific: Increasing innovation
Asia Pacific is expected to show significant growth in the inhaled nitric oxide delivery systems market during the forecast period. The growth of the market is increasing owing to the rising penetration of the different types of respiratory disease, including chronic obstructive pulmonary disease (COPD), neonatal respiratory distress syndrome (RDS), and others, which are driving the demand for nitric oxide supplements for the treatment of such types of disease is driving the demand for the inhaled nitric oxide delivery systems. The increasing investment by regional countries such as India, China, and Japan in improving healthcare infrastructure is accelerating the growth of the market across the region.
China: Large and growing patient base
China has a huge population, which, in conjunction with an increasing prevalence of respiratory diseases and an aging population, creates a considerable demand for treatments such as inhaled nitric oxide. The government mainly focuses on enhancing medical care access and quality, together with initiatives to modernize healthcare infrastructure.
Europe: Strong healthcare systems and infrastructure
Europe is significantly growing in the market a strong healthcare substructure, a growing prevalence of respiratory diseases, supportive regulatory policies, and a focus on healthcare innovation and research. The region benefits from a vigorous government framework, such as the CE Mark, which confirms high standards, and a well-recognized medical device sector. The region vigorously invests in clinical trials, medical research, and the advancement of novel technologies.
UK: Established healthcare infrastructure
The UK has been a key player in the research and development of iNO therapy, including the creation of clinical guidelines for its use in adult intensive care. A focus on enhancing patient results and a favorable reimbursement environment supports the adoption of costly, progressive treatments such as iNO.
Inhaled Nitric Oxide Delivery Systems Market- Value Chain Analysis
- R&D: The major R&D processes in the inhaled nitric oxide (iNO) delivery systems market encompass a multi-stage strategy from initial concept and design to widespread clinical trials and government approval, focused on enhancing safety, portability, and affordability.
Key Players: Linde and Air Liquide Healthcare - Clinical Trials: Major clinical trials in the inhaled nitric oxide (iNO) delivery systems industry are investigating its use in treating different conditions, including pulmonary hypertension (PH) associated with diseases
Key Players: Getinge and Vero Biotech - Patient Services:It involves clinical care and support, including respiratory management in intensive care and neonatal units, advanced monitoring, patient and family education, and home healthcare services.
Key Players: Mallinckrodt Pharmaceuticals
Top Vendors in the Inhaled Nitric Oxide Delivery Systems Market & Their Offerings
|
Company |
Headquarters |
Key Strengths |
Latest Info (2025) |
|
Mallinckrodt Pharmaceuticals |
United Kingdom |
Deep regulatory expertise |
Mallinckrodt Announced an Expanded Rollout of the INOmax EVOLVE DS Delivery System in U.S. Hospitals |
|
Getinge AB |
Sweden |
Strong financial performance |
Getinge and Philips have joined forces to offer hospitals an integrated anesthesia workstation for the operating room (OR). |
|
VERO Biotech |
United States |
User-centric design and operational efficiency |
It significantly focuses on the design, advancement, and commercialization of ground-breaking inhaled nitric oxide delivery systems to address the unmet healthcare needs of patients. |
|
Linde plc |
United Kingdom |
Largest industrial gas company |
Linde Healthcare is committed to quality care and patient safety, supported by pharmaceutical and medical device regulations and standards. |
|
Beyond Air, Inc. |
New York |
Logistical Efficiency |
Beyond Air is a clinical-stage medical device and biopharmaceutical company using nitric oxide to treat respiratory and other types of diseases. |
Recent updates on Inhaled Nitric Oxide Delivery Systems Market
- Advancements in iNO delivery systems enhance respiratory care
On 10 March 2025, due to the growing emphasis on respiratory therapies, the market for inhaled nitric oxide (iNO) delivery systems saw significant growth. Better patient safety and convenience are being achieved through the creation of small, easy-to-use devices with built-in monitoring features. In hospital and home care settings, these innovations are promoting broader adoption, especially when it comes to acute respiratory conditions.
- Rising neonatal care needs fuel market expansion
By April 2025, particularly in neonatal care, there was an increasing need for iNO delivery systems. A greater reliance on iNO therapy for prompt and efficient intervention has resulted from rising rates of respiratory complications in neonates. Clinical research and delivery technology developments are anticipated to further bolster market presence in a range of healthcare settings.
Latest Announcement by Industry Leaders
- In November 2024, Mallinckrodt plc, a leading specialty pharmaceutical company, was awarded as the winner of the Extracorporeal Immunomodulation Award (EIA). The award is given for the contribution to the optimal timing for extracorporeal photopheresis (ECP) initiation and for assessing its efficiencies when integrated with other graft versus host disease (GvHD) therapies.
Recent Development
- In January 2025, Mallinckrodt Pharmaceuticals expanded its iNO delivery system portfolio with the launch of a new portable device designed for both hospital and home care use. The system features advanced monitoring and a user-friendly interface for enhanced treatment safety.
- In February 2025, GE Healthcare introduced an upgraded iNO delivery platform integrating AI-driven analytics to help clinicians optimize dosage and monitor patient response in real time.
- In March 2025, Dräger partnered with healthcare institutions to pilot a new iNO system that integrates directly with ventilators, streamlining clinical workflows and reducing the need for standalone units.
- In April 2025, Beyond Air received FDA approval for its LungFit™ PH system—an innovative device that generates nitric oxide from ambient air, eliminating the need for gas cylinders and improving mobility and safety in treatment.
- In October 2024, Mallinckrodt plc, a leading specialty pharmaceutical company, INOmax EVOLVE™ DS delivery system for the delivery of INOmax (nitric oxide) gas for inhalation cleared by the U.S. Food and Drug Administration (FDA).
- In January 2023, VERO Biotech Inc., a commercial-stage healthcare organization focusing on the development of the neonatal intensive care and the acute care hospital community, announced its FDA approval for the tankless inhaled nitric oxide (iNO) delivery system.
- In October 2024, Beyond Air, Inc., a commercial-stage medical device and biopharmaceutical company that works on the endogenous and exogenous nitric oxide (NO), partnered with Healthcare Links, a healthcare advisory group for the expansion of the Beyond Air's LungFit PH system with entry into the Integrated Delivery Networks (IDNs) and Group Purchasing Organizations (GPOs) across the United States.
Segments Covered in the Report
By Type
- Pediatric
- Adult
By Product
- Disposables
- System
By Application
- Hypoxic Respiratory Failure (HRF)
- Acute Hypoxemic Respiratory Failure (AHRF)
- Others
By End-user
- Hospitals
- Ambulatory Centers
- Clinics
By Region
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting