Cell Therapy Market Size to Attain USD 83.78 Billion by 2032
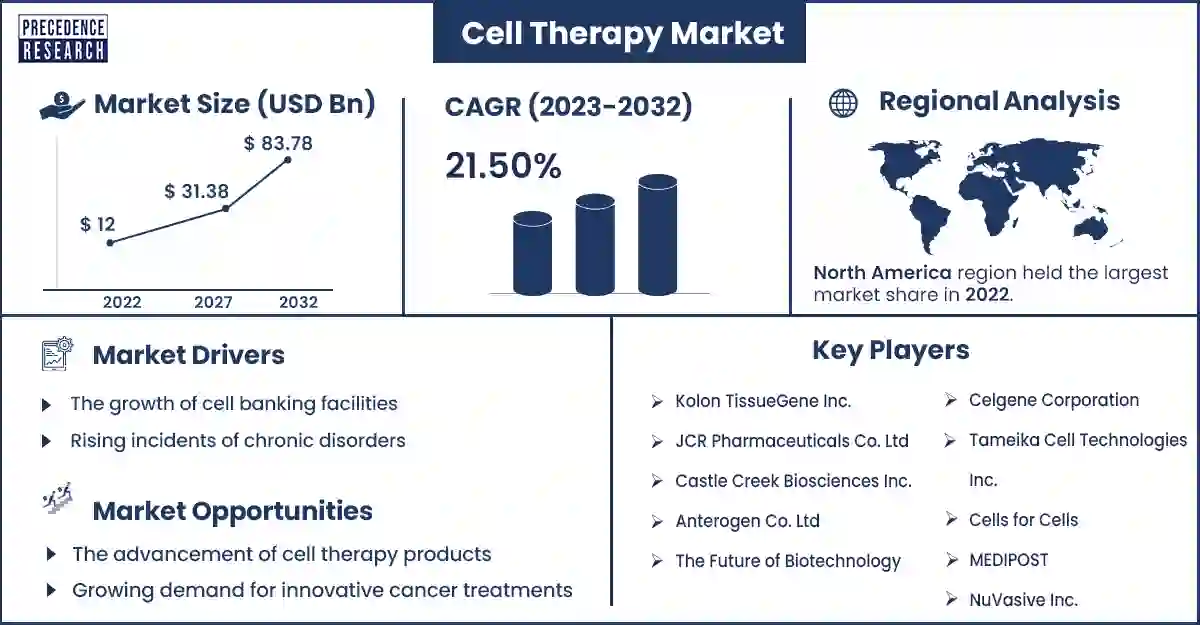
The global cell therapy market size surpassed USD 12 billion in 2022 and is expected to attain around USD 83.78 billion by 2032, growing at a CAGR of 21.50% from 2023 to 2032.
Market Overview
The cell therapy market is a rapidly evolving sector within the field of regenerative medicine, offering promising treatment options for a wide range of diseases and conditions. Cell therapy (also called cellular therapy, cell transplantation, or cytotherapy) is a therapy in which viable cells are injected, grafted, or implanted into a patient in order to effectuate a medicinal effect, for example, by transplanting T-cells capable of fighting cancer cells via cell-mediated immunity in the course of immunotherapy, or grafting stem cells to regenerate diseased tissues.
The global cell therapy market has experienced significant growth in recent years and is projected to continue expanding at a robust pace. Factors driving market growth include the increasing prevalence of chronic diseases, the growing aging population, advancements in cell therapy technologies, and rising investment in research and development. The market encompasses a diverse range of stakeholders, including biotechnology companies, pharmaceutical companies, academic research institutions, and contract manufacturing organizations (CMOs). The market holds immense promise for revolutionizing the treatment landscape across various diseases and conditions, with ongoing research, innovation, and investment driving continued growth and advancement in the field.
Regional Snapshots
North America dominated the cell therapy market in 2023. North America is a leading region in the market, driven by factors such as a robust biotechnology industry, advanced healthcare infrastructure, strong regulatory framework, and significant investment in research and development. The United States and Canada are the primary contributors to the North American market, with a concentration of biotechnology companies, academic research institutions, and healthcare facilities engaged in cell therapy research and development. Cell therapy companies in North America focus on developing innovative therapies targeting oncology, neurology, cardiology, and autoimmune diseases. The region has witnessed notable advancements in CAR T-cell therapies, mesenchymal stem cell (MSC) therapies, and regenerative medicine applications. Regulatory agencies such as the US Food and Drug Administration (FDA) and Health Canada play a crucial role in overseeing the development, approval, and commercialization of cell therapy products.
- Cellino Biotech, an autonomous cell treatment manufacturing firm, obtained a first investment round of $80 million in January 2022.
- Cellino wants to make stem cell-based medicines more accessible with the goal of building the first self-contained human cell foundry by 2025.
The Asia-Pacific region is a rapidly growing market for cell therapy, driven by factors such as increasing healthcare expenditure, rising prevalence of chronic diseases, growing biotechnology industry, and supportive regulatory reforms. Countries such as Japan, China, South Korea, and Australia are key players in the Asia-Pacific cell therapy market, with a focus on innovation, investment, and strategic collaborations in the field of regenerative medicine. Asian companies are at the forefront of developing cell therapy products targeting a wide range of diseases, including cancer, neurodegenerative disorders, diabetes, and cardiovascular diseases.
Cell Therapy Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 14.52 Billion |
| Projected Forecast Revenue by 2032 | USD 83.78 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 21.50% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Growth of cell banking
The growth of cell banking facilities allows for the storage and preservation of various cell types, including stem cells, immune cells, and other therapeutic cells. This accessibility fosters research and development by providing researchers and companies with a readily available source of cells for experimentation and therapy development. Cell banks play a crucial role in facilitating research by providing a consistent and standardized source of cells for experimentation in the cell therapy market. This enables researchers to conduct studies more efficiently and effectively, ultimately expediting the development of novel cell therapies. Cell banking also serves as a form of insurance against cell loss or contamination, ensuring that valuable cell lines are preserved for future use. This mitigates the risk associated with cell therapy development and production, thereby enhancing the reliability and robustness of cell-based treatments.
Allogenic therapies
Allogenic cell therapies, which utilize cells from a healthy donor instead of the patient themselves, offer scalability and cost-effectiveness compared to autologous therapies. This is because allogeneic therapies can be manufactured in large batches from a single donor, reducing production costs and enabling broader patient access. Allogenic cell therapies eliminate the need for patient-specific cell processing, simplifying manufacturing processes and reducing the time required to produce therapeutic doses. This enhances efficiency and scalability in manufacturing, making allogeneic therapies more attractive for commercialization. These therapies have the potential to be developed as off-the-shelf products that can be readily available for patient use, eliminating the need for personalized manufacturing. These address logistical challenges associated with autologous therapies and expand the reach of cell-based treatments to a larger patient population. This therapy contributes to the growth and advancement of the cell therapy market.
Restraints
Pre-clinical safety and efficacy profile
Despite advancements, there can still be concerns regarding the safety profile of cell therapies, particularly in the preclinical stages. Ensuring the safety of these therapies is paramount to gaining regulatory approval and public trust. Demonstrating the efficacy of cell therapies in pre-clinical studies can be complex. Variability in patient responses, inconsistency in manufacturing processes, and challenges in standardizing treatment protocols can all contribute to efficacy uncertainties.
Market knowledge gap
Limited understanding in many stakeholders, including healthcare providers, patients, and payers, may have a limited understanding of cell therapy technologies, their mechanisms of action, and their potential benefits and risks. This knowledge gap can hinder market uptake and acceptance. Efforts to educate key stakeholders about the potential of cell therapies and their role in addressing unmute medical needs are essential for market expansion. This includes providing clear, accurate information about the science behind these therapies, their applications, and their potential outcomes.
Opportunities
Advanced products
The advancement of cell therapy products involves various aspects, including technological innovation, improved manufacturing processes, and enhanced delivery methods. Companies investing in research and development to create more sophisticated and effective cell therapy products have the potential to capture significant market share. This could include the development of next-generation cell therapies with improved targeting, reduced side effects, and enhanced efficacy. Additionally, advancements in tissue engineering and regenerative medicine contribute to the growth of the advanced products segment in the cell therapy market.
CAR-T cell therapies
Chimeric Antigen Receptor T-cell (CAR-T) therapy has emerged as a revolutionary approach to cancer treatment. It involves genetically engineering a patient’s own T-cells to express chimeric antigen receptors, enabling them to recognize and attack cancer cells with precision. CAR-T therapies have shown remarkable success in treating certain types and improving their efficacy and safety profiles. Companies involved in CAR-T cell therapy development stand to benefit from the growing demand for innovative cancer treatments and the potential to transform patient outcomes.
RNA therapies
RNA-based therapies represent a promising frontier in the field of medicine. RNA therapies include approaches such as RNA interface (RNAi) and mRNA-based therapies, which can be utilized in cell therapy to modulate gene expression, manipulate cellular processes, or deliver therapeutic payloads. The RNA therapy market is expanding rapidly due to its potential to target previously undruggable diseases and provide personalized treatment options. In the context of the cell therapy market, RNA-based technologies can be employed to enhance the functionality of therapeutic cells, improve their survival and engraftment, or even direct their differentiation into specific cell types.
Recent Developments
- In February 2024, human serum AB product for the global cell therapy market launched by GeminiBio.
- In December 2023, rare disease drug development has grown exponentially in recent years and continued to expand on this trend. While many challenges remain, the increased focus on developing treatments for rare diseases provides hope for unveiling a growing number of solutions in the coming years.
Key Players in the Market
- Kolon TissueGene Inc.
- JCR Pharmaceuticals Co. Ltd
- Castle Creek Biosciences Inc.
- Anterogen Co. Ltd
- The Future of Biotechnology
- Celgene Corporation
- Tameika Cell Technologies Inc.
- Cells for Cells
- MEDIPOST
- NuVasive Inc.
Market Segmentation
By Use Type
- Clinical use
- By Therapeutic Area
- Malignancies
- Muscoskeletal Disorders
- Autoimmune Disorders
- Dermatology
- Cardiovascular
- Ocular
- Wounds and injuries
- Others
- By Cell Type
- Stem Cell Therapies
- BM, Blood, & Umbilical cord-derived Stem Cells
- Adipose-derived cells
- Others
- Non-stem Cell Therapies
- Research use
By Therapy Type
- Autologous Therapies
- Allogenic Therapies
By End User
- Hospitals
- Diagnostic centers
- Clinics
- Research institutes
- Regenerative medicine centers
- Others
By Technology
- Viral Vector Technology
- Genome Editing Technology
- Somatic Cell Technology
- Cell Immortalization Technology
- Cell Plasticity Technology
- Three-dimensional Technology
Buy this Research Report@ https://www.precedenceresearch.com/checkout/1700
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308