Minimal Residual Disease (MRD) Testing Market Revenue to Attain USD 4.22 Bn by 2033
Minimal Residual Disease (MRD) Testing Market Revenue and Trends 2025 to 2033
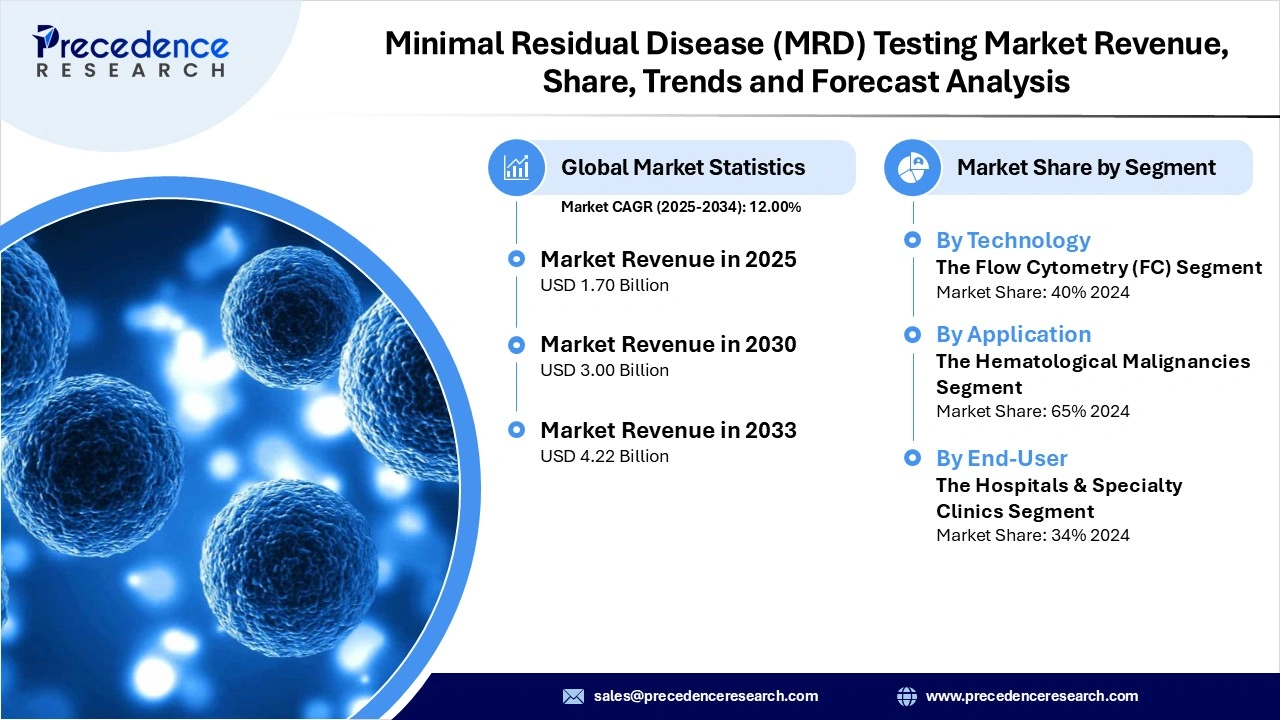
The global minimal residual disease (MRD) testing market revenue reached USD 1.70 billion in 2025 and is predicted to attain around USD 4.22 billion by 2033 with a CAGR of 12.00%. This market is growing due to the increased adoption of ultra-sensitive molecular diagnostics and growing integration of MRD-guided treatment pathways across oncology.
What factors supporting the growth of the minimal residual disease (MRD) testing market?
The advent of next-generation sequencing (NGS), digital droplet PCR, and immune repertoire profiling has dramatically enhanced analytic sensitivity and assay throughput. This allows for the detection of even a single cancer cell among millions of healthy ones. Strong evidence in hematologic cancers links minimal residual disease (MRD) negativity with extended progression-free (PFS) and overall survival (OS), leading to revised clinical guidelines and increased payer coverage. Diagnostic and biotechnology companies are working with cancer centers and labs to scale validation, standardize workflows, and automate processes, thereby reducing the cost per test.
The rising incidence of hematologic malignancies, expanding patient survivorship, and the shift toward minimally invasive liquid biopsies are also key drivers of market growth. Recent regulatory approvals and expanding reimbursement coverage in major markets are easing clinical adoption, improving affordability, and broadening access to MRD testing.
Segment Insights:
- By technology, the flow cytometry segment dominated the market in 2024, owing to its high-throughput capabilities, well-established clinical workflows, and superior analytical sensitivity for detecting MRD in hematologic malignancies.
- By application, the hematological malignancies segment led the market in 2024, due to the clinical validation of MRD testing in various leukemias and lymphomas, where it informs remission status and guides treatment de-escalation decisions.
- By end-user, the hospitals & specialty clinics segment held the largest market share in 2024, because of their high-volume oncology caseloads, integrated multidisciplinary care teams, in-house laboratories, and direct application of MRD results into intensive treatment and monitoring protocols.
Regional Insights:
North America dominated the global minimal residual disease (MRD) testing market in 2024, driven by early adoption of precision oncology, robust reimbursement systems, and the presence of leading diagnostic and biotech firms. The U.S. is a major contributor to the market, benefiting from a strong clinical trial infrastructure, high healthcare expenditures relative to other regions, and a favorable regulatory climate. Canada's public healthcare system is gradually integrating MRD into its cancer follow-up strategies. Furthermore, a concentration of reference laboratories and diagnostic networks accelerates the introduction of new technologies to the market.
Asia Pacific is expected to experience the rapid growth in the market, fueled by rising cancer incidence, enhancements in healthcare infrastructure, and the widespread adoption of molecular diagnostics, which collectively generate strong demand. Moreover, countries such as China, India, Japan, and South Korea are making significant investments in precision medicine and liquid biopsy technologies. Government support and funding for genomic diagnostics will boost the use of MRD assays. Additionally, growing oncologist awareness of MRD, along with its gradual inclusion in acceptable reimbursement schemes, is likely to sustain the market growth.
Minimal Residual Disease (MRD) Testing Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 1.70 Billion |
| Market Revenue by 2033 | USD 4.22 Billion |
| CAGR from 2025 to 2033 | 12.00% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Recent Development:
- On April 22, 2025, Invivoscribe’s LabPMM platform received New York State approval for its FLT3-ITD MRD assay, enabling ultra-sensitive NGS-based MRD detection in acute myeloid leukemia to guide targeted therapy decisions.
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/7039
You can place an order or ask any questions, please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344