Nasal Polyposis Treatment Market Revenue to Attain USD 8.61 Bn by 2033
Nasal Polyposis Treatment Market Revenue and Trends 2025 to 2033
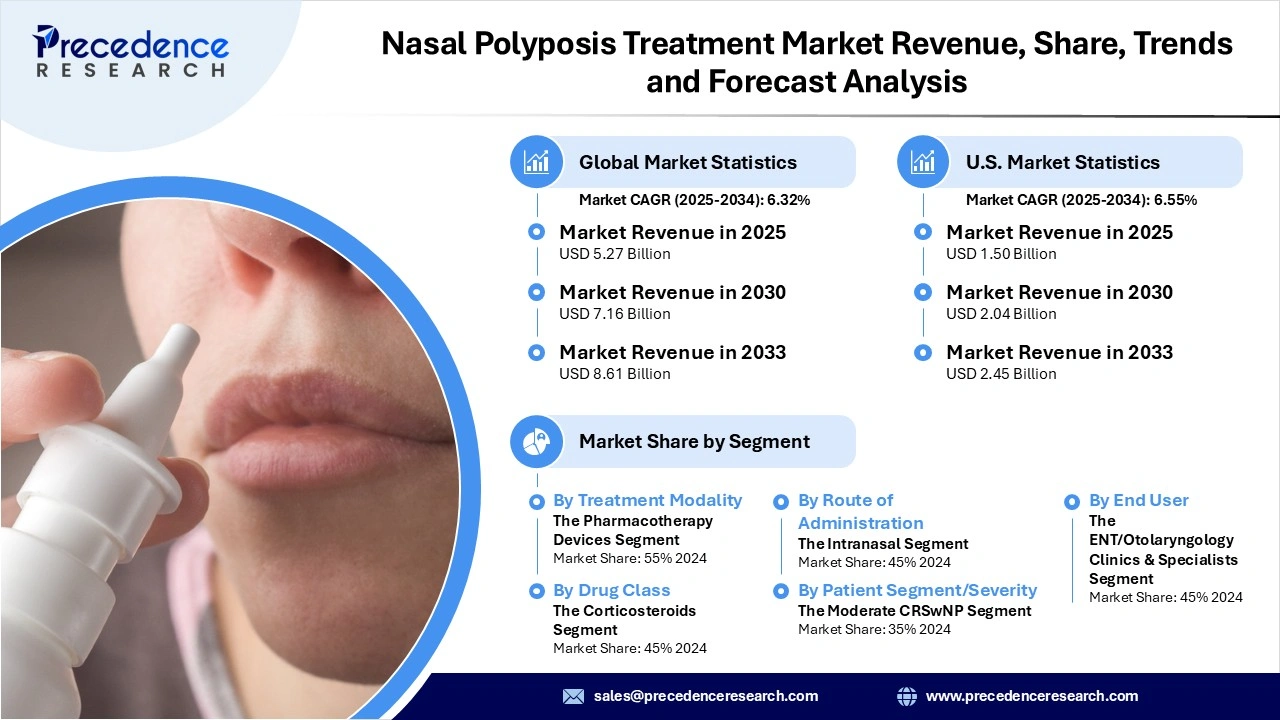
The global nasal polyposis treatment market revenue surpassed USD 5.27 billion in 2025 and is predicted to attain around USD 8.61 billion by 2033, growing at a CAGR of 6.32%. This market is growing because of rising CRSwNP incidence, rapid adoption of biologic therapies, and a broadening pipeline of non-surgical treatment options.
Key Drivers Enabling the growth of this market
The market for treating nasal polyps will mainly benefit from the increasing incidence of chronic rhinosinusitis with nasal polyposis (CRSwNP), particularly in older adults, underscoring a rapidly growing unmet need. Therapeutic advances in biologic and precision medicine, particularly interleukin-targeting approaches, are delivering more personalized and consistent control of inflammation than prior treatment with corticosteroids. Fast-tracked approval processes within regulatory agencies are accelerating the launch of new therapies and reducing the scope for surgical interventions. Access to Ear, Nose, Throat (ENT) specialists has increased, allowing improved diagnostic processes (e.g., nasal endoscopy), and patient engagement with nasal health and chronic sinuses has increased, resulting in a broader treatment-seeking patient population. These observed changes are significant market drivers overall.
Segment Insights
- By Treatment Modality, pharmacotherapy devices dominated the market in 2024, due to their proven clinical efficacy, widespread acceptance by physicians, and their ability to provide non-surgical relief in patients with nasal polyps.
- By Drug Class, Corticosteroids are the dominant segment of the market, owing to their long history of reducing inflammation, improving airflow of the nose, and being first line in many treatment guidelines.
- By Route of Administration, the intranasal segment dominated the market, due to its direct delivery to the nasal mucosa, greater patient convenience, and rapid relief of local symptoms with minimal systemic exposure.
- By Patient Segment/Severity, the moderate CRSwNP segment is dominating the market, as patients with moderate CRSwNP usually benefit the most from treatment with ongoing medical therapy prior to biologics or surgery.
- By Prescriber/End User, ENT and otolaryngology clinics will dominate the market, as diagnosticians of nasal polyps and experts in treatment options of nasal polyps.
Regional Insights
North America maintains its position as the leading regional market for nasal polyposis management, driven by its advanced clinical environment, rapid adoption of novel biologic therapies, and extensive access to ENT physicians. Furthermore, strong reimbursement pipelines, significant research participation, and trend awareness of chronic sinus disease adds to the region's leading market position.
Asia-Pacific is the fastest-growing regional market, driven by rapid healthcare modernization, expanded specialty ENT services, and increased patient awareness of nasal polyposis symptoms. While the improvement of healthcare spending, diagnostics, and acquisition of treatment across regional leaders have continued to accelerate treatment, pharmaceutical investment and government initiatives aimed to bolster respiratory and allergy care has played a major role in the region robust growth.
Nasal Polyposis Treatment Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 5.27 Billion |
| Market Revenue by 2033 | USD 8.61 Billion |
| CAGR from 2025 to 2033 | 6.32% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Recent Developments
- October 17, 2025, FDA Approval of Tezspire: Amgen and AstraZeneca announced that the U.S. FDA approved TEZSPIRE (tezepelumab-ekko) for add-on maintenance treatment of inadequately controlled CRSwNP in patients aged 12 and older. TEZSPIRE is the first biologic targeting thymic stromal lymphopoietin (TSLP) for nasal polyps.(Source: https://www.amgen.com)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/7115
You can place an order or ask any questions, please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344