Pharmaceutical Rapid Microbiology Testing Market Revenue to Attain USD 4.80 Bn by 2033
Pharmaceutical Rapid Microbiology Testing Market Revenue and Trends 2025 to 2033
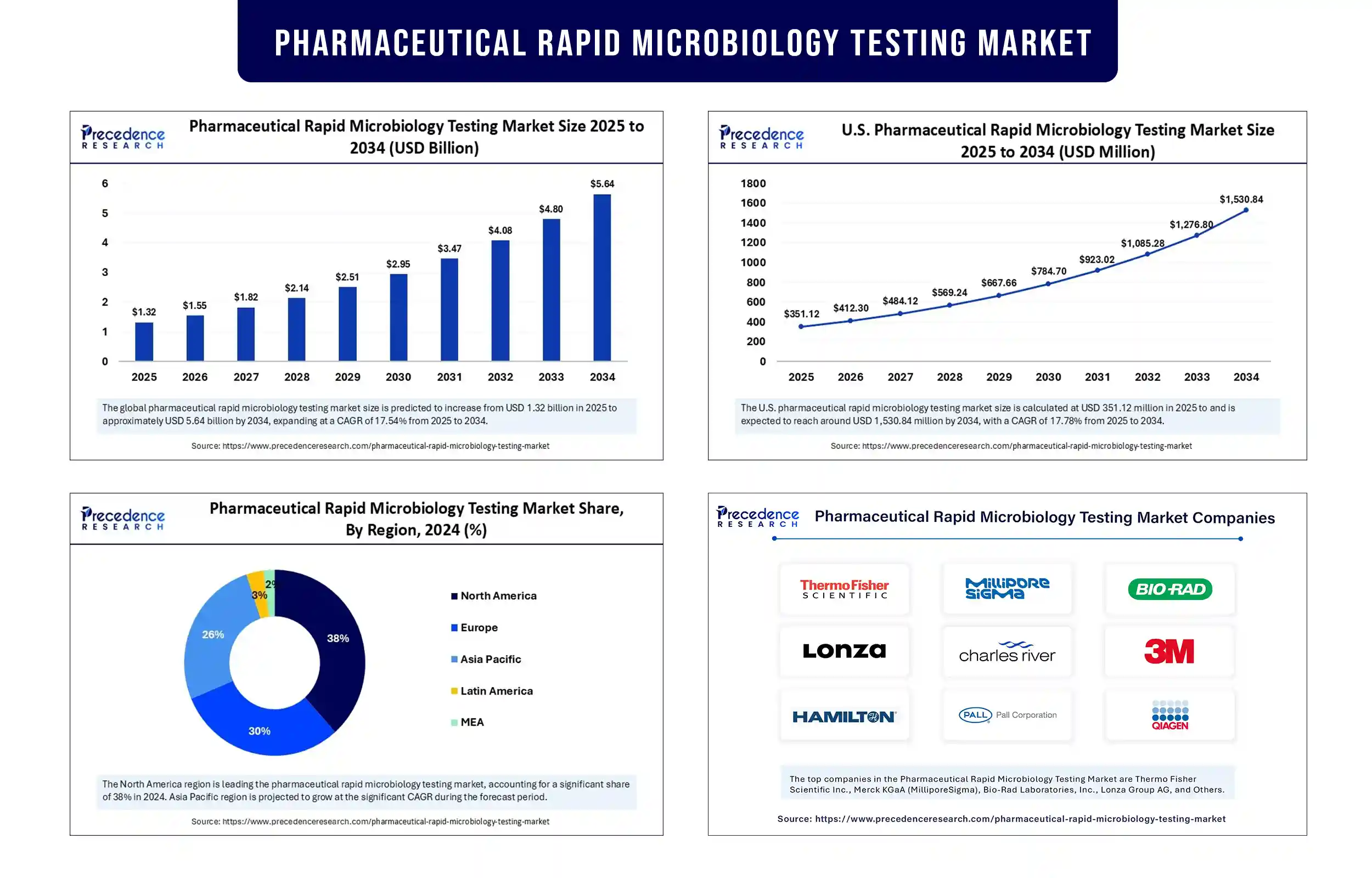
The global pharmaceutical rapid microbiology testing market revenue surpassed USD 1.32 billion in 2025 and is predicted to attain around USD 4.80 billion by 2033, growing at a CAGR of 17.54%. The growth of the market is attributed to the increasing demand for faster, more accurate microbial testing to ensure drug safety and quality. The rising discovery and development of novel drugs further contributes to market expansion.
Market Overview
The pharmaceutical rapid microbiology testing market is experiencing robust growth, fueled by several factors. A key driver is the rising need for quicker, more precise microbial detection in drug manufacturing, spurred by regulatory requirements from agencies like the FDA and EMA. Rapid microbiological solutions offer advantages in sensitivity, real-time results, and automation compared to time-consuming, error-prone traditional methods. Furthermore, increased pharmaceutical production, particularly due to global health crises, contributes to this growth. Moreover, the rising production of biologics and advances in next-generation sequencing support market expansion.
Segment Insights
- By technology type, the polymerase chain reaction (PCR) segment continues to dominate the market due to its high degree of sensitivity and accuracy in detecting pathogens. The rising demand for rapid testing is likely to ensure the long-term growth of the segment.
- By product type, the instruments segment dominated the market in 2024. This is mainly due to the increased demand for endotoxin analyzers, flow cytometers, and PCR systems that are essential for carrying out microbial detection procedures. The rising need for automation and efficient contamination detection solutions further supports segmental growth.
- By application, the biopharmaceuticals segment dominates the market, driven by the increasing production of biologics, strict standards for quality, and the need for rapid and reliable contamination detection solutions.
- By microorganism type tested, the bacteria segment led the market in 2024, driven by an urgent need for the rapid detection of bacterial contaminants during the manufacturing of drugs and quality assurance testing.
- By end-user, the pharmaceutical and biotechnology companies segment dominated the market in 2024. These companies are major adopters of microbiology testing due to stringent regulations regarding the quality and safety of pharmaceutical products.
- By testing type, the sterility testing segment continues to dominate the market, driven by the rising demand for sterile pharmaceuticals. In addition, strict regulations regarding pharmaceuticals’ quality and sterility augment the segment.
Regional Insights
North America registered the pharmaceutical rapid microbiology testing market by holding the largest share in 2024, driven by stringent regulations regarding the safety, quality, and efficacy of pharmaceutical products. The region is home to a large number of leading pharmaceutical and biotech companies, leading to the increasing production of pharmaceutical and biologics, which significantly drives the adoption of microbiology testing.
Asia-Pacific is the fastest-growing region. Rising government initiatives to improve healthcare systems and increased awareness about microbiological safety are likely to drive market growth in the region. The rising development of novel drugs supports regional market growth.
Pharmaceutical Rapid Microbiology Testing Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 1.32 Billion |
| Market Revenue by 2033 | USD 4.80 Billion |
| CAGR from 2025 to 2033 | 17.54% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Development
- In January 2024, Rapid Micro Biosystems, Inc. announced that Samsung Biologics has selected the Growth Direct platform to automate its microbial quality control processes to deliver increased efficiency, more robust data integrity, and scalable quality control operations.
(Source: https://investors.rapidmicrobio.com)
Pharmaceutical Rapid Microbiology Testing Market Key Players
- Thermo Fisher Scientific Inc.
- Merck KGaA (MilliporeSigma)
- Bio-Rad Laboratories, Inc.
- Lonza Group AG
- Charles River Laboratories International, Inc.
- 3M Company
- Hamilton Company
- Microbiological Solutions (a part of Pall Corporation)
- Pall Corporation
- Pall Life Sciences
- Qiagen N.V.
- PerkinElmer, Inc.
- Eurofins Scientific
- IDEXX Laboratories, Inc.
- BioMérieux SA
- Neogen Corporation
- Agilent Technologies, Inc.
- Nova Biomedical Corporation
- LuminUltra Technologies Ltd.
- Accelerate Diagnostics, Inc.
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6332
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344