Psychedelic API Market Revenue to Attain USD 14.70 Bn by 2033
Psychedelic API Market Revenue and Trends 2025 to 2033
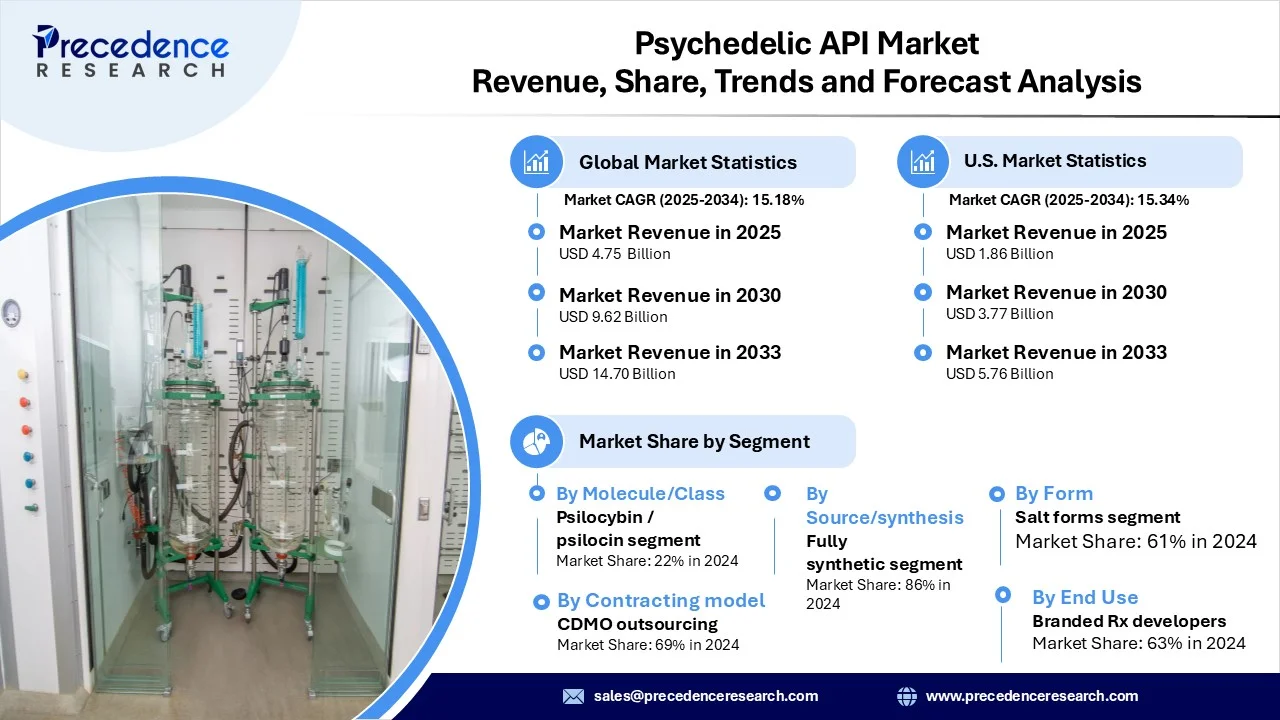
The global psychedelic API market revenue reached USD 4.75 billion in 2025 and is predicted to attain around USD 14.70 billion by 2033 with a CAGR of 15.18%. This market is growing due to breakthroughs in clinical research, evolving regulatory frameworks, and rising demand for innovative neuropsychiatric treatments, together driving strong investment and robust development pipelines.
What are the key drivers enabling the growth in the psychedelic API market?
The market is growing due to a convergence of scientific, regulatory, and commercial factors. Rising rates of mental health conditions like treatment-resistant depression and PTSD are creating urgent demand for new therapies. Positive clinical trial results for compounds such as ketamine, esketamine, psilocybin, and MDMA have demonstrated safety and efficacy, fueling further R&D and investment.
Regulatory shifts, particularly in North America, are enabling compassionate use, fast-track approvals, and controlled studies. Major pharmaceutical and biotech firms are entering the space via partnerships, acquisitions, and CDMO deals, bringing capital and commercialization expertise. Growing public acceptance is also accelerating medical adoption and scale-up of psychedelic API production.
Segment Insights:
- By molecule/class, the ketamine/esketamine segment dominated the market in 2024, driven by existing approvals (e.g., Spravato) and an expanding development pipeline.
- By source/synthesis route, the fully synthetic segment led in 2024 due to its advantages in purity, scalability, and regulatory compliance compared to plant-derived alternatives.
- By form/solid-state & grade, the salt forms segment held the largest share in 2024, as salt APIs offer greater stability, better solubility control, and enhanced formulation flexibility compared to base forms.
- By contracting model, the CDMO outsourcing segment dominated the market, with many biotech and pharma companies preferring to outsource specialized psychedelic API development and manufacturing to expert contract partners.
- By end-use, the branded prescription drug developers/sponsors segment led the market, as these developers drive most innovation and investment in proprietary drug development. They hold exclusive rights to novel compounds, invest heavily in clinical trials, and focus on creating patented, high-value therapies, which results in significant demand for specialized psychedelic APIs
Regional Insights
North America dominated the psychedelic API market with the largest share in 2024, driven by a strong clinical trial infrastructure, a robust biotech ecosystem, and progressive regulatory frameworks. The U.S. leads in investment, intellectual property activity, and early adoption of regulatory pathways supporting psychedelic APIs. This regional dominance is further reinforced by the presence of key industry players, significant funding sources, and a well-established pharmaceutical landscape.
On the other hand, Asia Pacific is emerging as the fastest-growing area, driven by developing neuroscience research hubs, increasing awareness of mental health, and the expansion of biotech capabilities. Updates to regulatory frameworks in parts of Asia, along with growing local funding and rising academic interest in psychedelics, are fueling this growth. Countries like China, Australia, and South Korea are leading the way by investing in clinical infrastructure and local active pharmaceutical ingredient (API) synthesis capabilities, positioning the region as a new frontier for development.
Psychedelic API Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 4.75 Billion |
| Market Revenue by 2033 | USD 14.70 Billion |
| CAGR from 2025 to 2033 | 15.18% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2025 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Recent Development:
- In August 2025, AbbVie acquired Gilgamesh Pharmaceuticals’ lead psychedelic candidate, bretisilocin, for up to USD 1.2 billion, strengthening its neuroscience portfolio and highlighting the pharmaceutical industry's renewed focus on psychedelic therapeutics. (Source- https://www.genengnews.com)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/7001
You can place an order or ask any questions, please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344