Viral Vectors-Based Gene Therapy for Non-Human Primates Market Revenue to Attain USD 4.73 Bn by 2033
Viral Vectors-Based Gene Therapy for Non-Human Primates Market Revenue and Trends 2025 to 2033
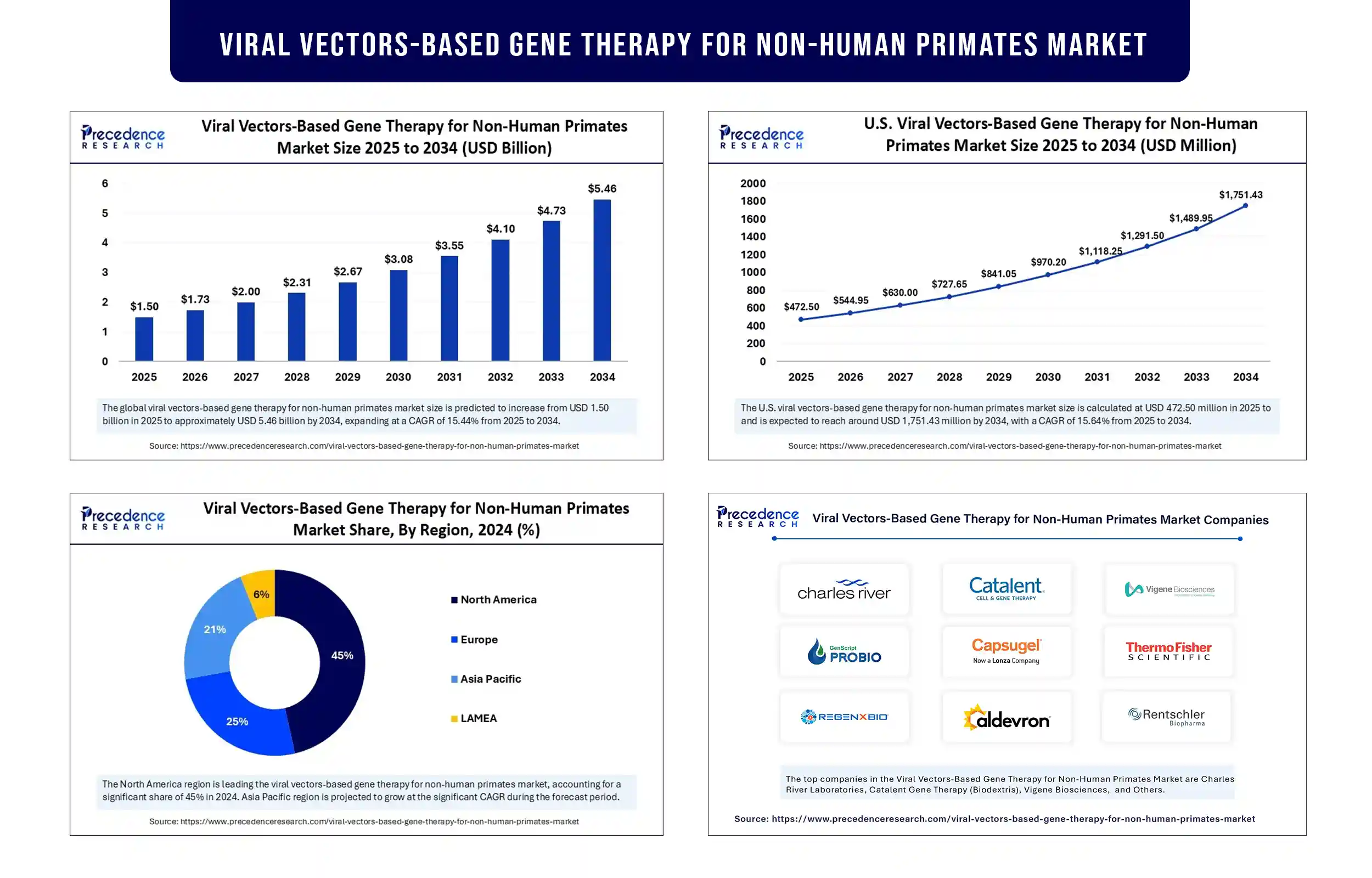
The global viral vectors-based gene therapy for non-human primates market revenue was valued at USD 1.50 billion in 2025 and is expected to attain around USD 4.73 billion by 2033, growing at a CAGR of 15.44% during forecast period. The growth of the market is attributed to the increasing demand for preclinical models that closely mimic human physiology in the development of advanced gene therapies.
What are the Key Factors Boosting the Growth of the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
The viral vectors-based gene therapy market for non-human primates is growing significantly due to the increasing demand for translational research models that closely mimic human disease biology. Key drivers include the rising research into rare and genetic disorders, funding for advanced biotechnology platforms, and the growing use of AAV and lentiviral vectors in preclinical studies. Supportive regulatory frameworks and partnerships between academic institutions and biopharma companies are further fueling this market growth. Moreover, advancements in vector technology and increasing demand for personalized medicine create immense growth opportunities for the market.
Segment Outlook
- By vector type, the adeno-associated virus (AAV) segment registered dominance in the market while holding the largest share in 2024. This is mainly due to its proven safety and efficacy profile and ability to provide robust gene delivery.
- By application, the ophthalmology (retinal gene therapy) segment led the market in 2024. This is mainly due to the increased research into the field of vision disorders. The proven effectiveness of viral vector-based gene therapy in treating age-related macular degeneration further bolstered segmental growth.
- By service type, the vector manufacturing & GMP-grade supply segment dominated the market in 2024, as researchers continue to do preclinical research with strict, quality-driven approaches.
- By end-user, the biopharma & gene therapy companies segment led the market for viral vectors-based gene therapy market in non-human primates in 2024, since they pioneer and innovate in the field with robust therapeutic research.
Regional Insights
North America registered dominance in the viral vectors-based gene therapy for non-human primates market in 2024. The U.S. is a major contributor to the market due to increased preclinical research studies and the presence of leading biotech and pharmaceutical companies. There is a high demand for personalized medicine, contributing to market growth. A supportive regulatory environment further supports market growth.
Asia Pacific is expected to grow at a significant rate in the coming years. This is mainly due to the expanding biotech industry in countries like China, Japan, South Korea, and India. The rising prevalence of rare and genetic diseases further drives the demand for gene therapies, which bolsters the growth of the market.
Viral Vectors-Based Gene Therapy for Non-Human Primates Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 1.50 Billion |
| Market Revenue by 2033 | USD 4.73 Billion |
| CAGR from 2025 to 2033 | 15.44% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Development
- In June 2025, Sarepta Therapeutics, Inc. announced that the rAAVrh74 viral vector used in the investigational gene therapy SRP-9003 (bidridistrogene xeboparvovec) for the treatment of limb-girdle muscular dystrophy type 2E/R4, has been granted platform technology designation by the U.S. FDA.
(Source: https://investorrelations.sarepta.com)
Viral Vectors-Based Gene Therapy for Non-Human Primates Market Key Players
- Charles River Laboratories
- Catalent Gene Therapy (Biodextris)
- Vigene Biosciences
- GenScript ProBio
- Lonza (Capsugel)
- Thermo Fisher Scientific
- REGENXBIO
- Aldevron (Danaher)
- Novasep / Rentschler
- Oxford Biomedica
- GE Healthcare (Cytiva)
- Wave Life Sciences
- BioIVT / BioIVD
- ICON plc
- Covance (Labcorp)
- Envigo (Inotiv)
- SAB Biotherapeutics
- Precision Nanosystems
- Battery-quality NHP Centers (e.g., SNPRC)
- Inotiv (formerly BioAnalytics)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6454
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344