What is the Alkaptonuria Therapeutics Market Size?
The global alkaptonuria therapeutics market size is accounted at USD 16.19 million in 2025 and predicted to increase from USD 17.36 million in 2026 to approximately USD 30.32 million by 2034, expanding at a CAGR of 7.22% from 2025 to 2034. The market growth is attributed to increasing research efforts, rising adoption of targeted therapies, and expanding rare disease awareness programs, driving early diagnosis and improved treatment accessibility.
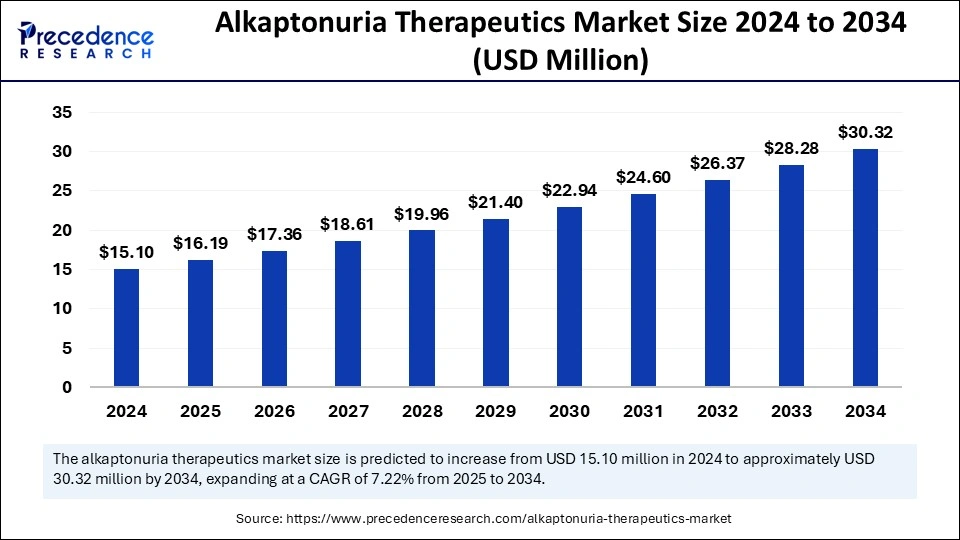
Alkaptonuria Therapeutics Market Key Takeaways
- North America dominated the alkaptonuria therapeutics market in 2024.
- Asia Pacific is projected to grow at the fastest CAGR in the coming years.
- By drug type, the nitisinone (p-hydroxyphenyl-pyruvate dioxygenase inhibitor) segment held the largest market in 2024.
- By drug type, the dietary supplements segment is expected to grow at a solid CAGR during the forecast period of 2025 to 2034.
- By route of administration, the oral segment accounted for a considerable share of it in 2024.
- By rote of administration, the parenteral segment is expected to grow at a highest CAGR in the studied years.
- By patient age group, the adult segment led the global market in 2024.
- By patient age group, the pediatric segment is projected to expand rapidly in the coming years.
- By distribution channel, the hospital pharmacies segment dominated the global market in 2024.
- By distribution channel, the online pharmacies segment is projected to grow at the fastest CAGR in the future years.
Impact of Artificial Intelligence (AI) on the Alkaptonuria Therapeutics Market
The therapeutic landscape for rare diseases experiences transformation through Artificial Intelligence systems as they speed up drug research and enhance clinical trials and diagnostic tests. Machine learning programs help scientists discover alkaptonuria drug candidates through automatic screening, which decreases development periods in the process. Such models examine large genetic study and biochemical pathway databases to discover new therapeutic targets that enhance treatment strategy precision.
Predictive analytics and AI-based imaging solutions help medical professionals detect concealed early signs of diseases that normal approaches miss. Research shows that the continuing development of AI expands its capability to drive targeted medicine innovations specifically for alkaptonuria treatment, which results in improved patient clinical results.
What Is Contributing to the Rapid Expansion of the Alkaptonuria Therapeutics Market?
The approval from regulatory authorities such as the European Medical Agency (EMA) and European Commission has dramatically improved alkaptonuria management by providing a therapeutic solution that stops the disease's progression. Furthermore, the future expansion of the alkaptonuria therapeutics market is projected from sustained attention to rare disease investigation and treatment development initiatives.
- The European Medicines Agency (EMA), together with the European Commission, authorized nitisinone for treating alkaptonuria form 2020, and this made the medication accessible to patients across Europe along the United Kingdom.
Nitisinone is a promising active pharmaceutical ingredient for the treatment of alkaptonuria. It has led to substantial market expansion in the field of alkaptonuria therapeutics since its approval as a medical treatment option. Nitisinone blocks the activity of 4-hydroxyphenylpyruvate dioxygenase (HPPD) enzyme to lower homogentisic acid accumulation, thus minimizing the tissue damage in alkaptonuria.
The Alkaptonuria therapeutics market internationally is gaining traction as awareness of rare metabolic disorders increases and novel enzyme-replacement therapies come into the scope of the clinical pipeline. Advancements in diagnostic technologies and expanded newborn screening programs have led to increased demand for ultra-orphan treatments. Pharmaceutical innovators have increased research activity for nitisinone-based formulations, and supportive regulations for orphan drugs have continued to provide a promising outlook. In sum, these factors are establishing a promising growth outlook for the care solutions for patients diagnosed with Alkaptonuria.
Alkaptonuria Therapeutics Market Growth Factors
- Increasing adoption of genetic testing is driving the alkaptonuria therapeutics market by enhancing early diagnosis and patient identification.
- Rare disease awareness programs led by governments and NGOs are fueling the market by improving early detection and expanding treatment accessibility.
- Advancements in biomarker research are propelling the market by refining treatment monitoring and disease progression assessment.
- Rising investments in orphan drug development are boosting the market as regulatory incentives and rare disease funding accelerate research.
- The development of enzyme replacement therapies is driving the market by creating long-term treatment solutions beyond nitisinone.
- A growing focus on personalized medicine is fueling the market by improving patient-specific treatment strategies and outcomes.
- AI integration in drug discovery is propelling the market by accelerating the identification of novel therapeutic compounds.
Market Outlook:
- Industry Growth Overview: The demand for Alkaptonuria treatment will likely increase with more clinical awareness and as new treatment modalities reach commercialization. Interest in orphan drug tax incentives will also boost reliable industry growth.
- Sustainability Trends: Industrial manufacturers have begun utilizing more eco-efficient production methodologies that limit waste through bio-chemical processing, as well as established digital diagnostics in a multi-modal approach, ensuring sustainable delivery of treatment for rare diseases.
- Global Expansion: Markets outside of the usual are being pursued by pharmaceutical companies, in conjunction with hospitals, patient advocacy, and government rare-disease programs, providing greater access globally to Alkaptonuria therapies.
- Startup Ecosystem: Although a still maturing process, more biotechs will enter the rare disease space with indications targeting gene therapy, metabolic pathways, and diagnostics that utilize artificial intelligence, which will help enhance the competitive landscape of treatment for Alkaptonuria.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 30.32 Million |
| Market Size in 2025 | USD 16.19 Million |
| Market Size in 2026 | USD 17.36 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 7.22% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Type, Route of Administration, Patient Age Group, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Drivers
Expanding awareness and advancements in early diagnosis
Growing awareness campaigns and diagnostic advancements are anticipated to improve early detection rates, enabling timely intervention. The expansion of awareness initiatives, together with enhanced diagnostic capabilities, results in earlier alkaptonuria diagnoses, thus enabling immediate medical intervention. The use of genetic screening programs, together with next-generation sequencing technologies, helps medical professionals detect alkaptonuria before serious complications occur.
Healthcare providers, along with patient advocacy groups and rare disease organizations, work together to teach people about alkaptonuria signs and its stage development. Government programs for newborn screening of metabolic diseases enhance diagnostic abilities, which enables health providers to intervene at an earlier stage. These advancements demonstrate worldwide dedication towards early detection and care of rare genetic conditions that benefit patients with alkaptonuria.
- NHS England established 2024 as the starting year for their groundbreaking initiative, which involves whole genome sequencing of 100,000 newborns to detect more than 200 genetic disorders in order to produce early medical results. This project was launched to improve early medical treatment accessibility to infants.
Populations Suffering from Rare Disorders, 2022-2024
| Year | Estimated Global Population |
| 2022 | 8.0 billion |
| 2023 | 8.1 billion |
| 2024 | 8.2 billion |
Restraint
Scarcity of approved therapies
The scarcity of approved therapies is expected to challenge treatment accessibility, leaving patients with limited pharmacological options. Pharmaceutical organizations refrain from investing in treatments for ultra-rare diseases because these conditions yield minimal potential for the alkaptonuria therapeutics market, which slows down discoveries in therapeutic development. Approval processes for rare disease treatments managed by regulatory agencies demand extensive clinical validations that extend the duration required for commercialization.
Opportunity
Increasing research and development initiatives
Increasing investments in research and development activities are projected to drive advancements in targeted therapies for alkaptonuria, which further creates immense opportunities for the players competing in the market. Pharmaceutical organizations, together with academic research institutions, develop enzyme replacement treatments and gene-editing methods, and drug repurposing techniques to heal metabolic flaws in alkaptonuria patients.
- The National Institutes of Health (NIH) showed its biomedical research commitment through a 4.7% funding increase reported in 2023 to amount to USD 34.92 billion.
Clinical evaluations of nitisinone therapy and new treatment compounds are continuing to expand for the purpose of reducing homogentisic acid accumulation and long-term complications prevention. New research on biochemical mechanisms enables healthcare providers to enhance therapy through precise medical methods that yield enhanced patient healing results. Alkaptonuria research receives increased emphasis on developing innovative treatments due to extensive research funding alongside promising clinical study results.
Drug Type Insights
The nitisinone (p-hydroxyphenyl-pyruvate dioxygenase inhibitor) segment dominated the alkaptonuria therapeutics market during the forecasting period. The therapeutic agent Nitisinone shows effectiveness in homogentisic acid reduction, as this substance functions as the main factor in alkaptonuria-related medical complications. The European Medicines Agency (EMA) formally approved Nitisinone as the first alkaptonuria therapy for its effectiveness and broader use of the treatment. Monitored clinical trials showed that nitisinone therapy reduced homogentisic acid in patients' urine samples, thereby confirming its medical benefit.
The dietary supplements segment is expected to grow at the fastest rate during the forecast period of 2025 to 2034. The growing trend is for patients, together with healthcare professionals, to center their disease management approach on nutritional practices alongside supplementation. Patients who take dietary supplements with vitamin C and antioxidants face a potential benefit of slowing the disease progression rate through their antioxidant effects on alkaptonuria-derived oxidative stress. The Council for Scientific and Industrial Research (CSIR) India suggests pharmacological advancements boost the demand for supplementary medicine as well as preventable healthcare services. The wide accessibility of dietary supplements that are purchased without a prescription contributes to their expected market dominance, as they reach a high number of patient communities.
Route of Administrations Insights
The oral segment held the largest revenue share of the alkaptonuria therapeutics market during the forecasting period. The oral pharmaceutical substance reduces the accumulation of homogentisic acid, which creates complications in alkaptonuria patients. The production expenses of oral dosage forms stay lower than those of parenteral or topical products as they become available to broader groups of patients. The segment has gained further market strength through the regulatory approval of nitisinone as an oral medication.
The parenteral segment is anticipated to grow with the highest CAGR in the studied years, owing to the ongoing enzyme replacement therapy research and development of gene therapies, which probably result in therapeutic delivery methods that require intravenous or subcutaneous administration. Medical delivery methods provide a direct pathway for therapeutic substances into the bloodstream, which enables swift drug effects with accurate dosing capability. Furthermore, the expanding use of sophisticated treatment approaches as they advance through the clinical testing process and gain regulatory clearances further fuels the segment in the coming years.
Patient Age Group Insights
The adult segment dominated the alkaptonuria therapeutics market due to the symptoms generally appearing after age thirty, resulting in increased diagnosis and treatment of these patients. Adults with homogentisic acid buildup in their system develop dark-pigmented connective tissues referred to as ochronosis, along with arthritic conditions that affect weight-bearing joints most severely. Medical intervention becomes essential as these progressively worsening symptoms increase the therapy requirements in the affected population.
- The alkaptonuria disease's occurrence rate across international populations shows a wide variation between each demographic, measured at 1 in 250,000 to 1 in 1,000,000, according to the NIH 2023 report.
The pediatric segment is projected to expand rapidly in the coming years. The identification of alkaptonuria through genetic screening and early diagnostic techniques shifts the market towards children, as these techniques allow doctors to identify alkaptonuria before serious symptoms occur. Early treatment methods, which combine nutritional adjustments and medical medications, work to halt disease progression or stop its advancement from the beginning. Healthcare professionals, along with parents, increase their early detection knowledge as the market demand for paediatric therapeutic solutions is growing.
Distribution Channel Insights
The hospital pharmacies segment held the largest share of the alkaptonuria therapeutics market during the forecasting period. The specialized treatments for alkaptonuria require healthcare professional supervision, thus leading to hospital pharmacies' market dominance. Hospital pharmacies keep a complete stock of unlikely disease medications to ensure quick access for their patient population. Hospital integrated care systems allow doctors to share information directly with pharmacy staff, which leads to improved patient outcomes and medication usage performance. Furthermore, the hospital pharmacies deliver specific patient education together with support services to assist individuals with managing their challenging condition of alkaptonuria.
The online pharmacies segment is projected to grow at the fastest rate in the future years, owing to the increasing growth rates of online pharmacies' availability. The merging of digital health platforms with home delivery convenience services causes this transition in the future. People living in remote locations gain better access to medications through online pharmacy services since these platforms distribute special pharmaceuticals unavailable at standard retail outlets. Online research allows patients to gain essential knowledge about their care options and assessment of prices, which helps them make smarter healthcare decisions. Furthermore, online pharmacies receive greater trust from consumers, as the regulatory organizations now establish safety guidelines for these virtual pharmaceutical facilities.
- As of 2024, 82% of WHO European Region member states have implemented digital prescription systems, facilitating the growth of online pharmacies.
Regional Insights
North America led the alkaptonuria therapeutics market, capturing the largest share in 2024, as the region holds advanced healthcare facilities together with robust regulatory structures and substantial funding for rare disease therapeutic research. The National Institutes of Health (NIH) maintains its ongoing support for metabolic disorder research, specifically for alkaptonuria, which advances specific treatment methods. It allocates approximately USD 3.5 billion annually to rare diseases research, facilitating the development of novel therapies for conditions.
Genetic screening programs and metabolic clinics located in the United States and Canada enable patients to get diagnosed early with the opportunity for timely treatments. The awareness campaigns initiated by rare disease organizations, including the National Organization for Rare Disorders (NORD), have improved both disease education alongside treatment accessibility for patients. North America maintained its position as leader in the alkaptonuria therapeutics market of 2024 due to these multiple market-promoting factors.
Asia Pacific is projected to host the fastest-growing alkaptonuria therapeutics market in the coming years. Genetic screening expansion throughout these countries improves alkaptonuria early diagnosis, which leads to faster interventions. The growth of patient access to treatment, together with therapeutic advancements, stems from government funding programs that back rare disease research. Furthermore, the partnership between international pharmaceutical businesses and local healthcare organizations creates stronger access to specialized medicines, which further facilitates the market in this region.
Asia Pacific: China & India Alkaptonuria Therapeutics Market Trends
In China and India, the alkaptonuria (AKU) therapeutics market is gaining momentum as more patients are identified and there's rising awareness about this rare metabolic disorder. Both countries are seeing increased investment in orphan drug development, spurred by government incentives and growing capacity in rare-disease R&D. The potential for nitisinone (which drastically reduces homogentisic acid levels) is a key driver, especially as local companies consider manufacturing or importing this repurposed therapy.
According to the NIH 2024 report, countries such as China and India have experienced annual healthcare expenditure growth rates of 8% and 7%, respectively, between 2019 and 2023, enhancing healthcare infrastructure and access to treatments.
How Is Latin America Responding to the Rising Demand for Alkaptonuria Treatments?
Latin America is experiencing a steady increase in diagnoses with improved genetic testing access and a healthcare professional group more aware of rare metabolic diseases. Several countries in the region, primarily Brazil, Mexico, and Argentina, are developing and integrating healthcare systems to allow patient access to enzyme-replacement therapies. Device partnerships with hospitals and pharmaceutical agents in the region are growing as well.
While therapy costs are high, rare disease programs funded by government agencies and patient advocate organizations will help reduce the time lag of treatments before coming to market.
Why Is Europe an Emerging Center for the Development of Alkaptonuria Therapeutics?
Europe continues to be a leading region in developing Alkaptonuria therapeutic products due to established biotechnology research clusters, orphan drug legislation, and significant investments into rare disease research. Countries such as the U.K., Germany, and France continue to establish advanced clinical trial infrastructure that supports emerging therapeutic treatments.
Through policies surrounding reimbursement, these countries have established patient registry systems to help drive early diagnosis and monitor treatment development of those with Alkaptonuria. Furthermore, Europe has established education training systems that can train on research centers and pharmaceutical companies, building on its location and establishment as a central market contributor.
Value Chain Analysis:
How Does the Value Chain Reinforce the Alkaptonuria Therapeutics Market?
The value chain of the Alkaptonuria therapeutics market includes drug discovery, clinical development, manufacturing, and distribution. Each stage of the value chain is heavily impacted by factors such as research funding and regulatory compliance. Dedicated pharmaceutical companies like Swedish Orphan Biovitrum (Sobi), InfaCare Pharmaceutical Corporation, and Pfizer are driving innovation through their investments in the next-generation metabolic therapy pipeline. Additionally, research institutions play a vital role, through exploratory biomarkers or coordination of sourcing sites for clinical trials.
Specialty manufacturers promote strict cGMP to ensure quality during enzyme-replacement (ER) and small-molecule therapy manufacturing. Distribution partners work closely with hospitals, rare disease centers, and online pharmacy networks to ensure patients receive timely access to their medication. The reinforcement of the overall candidacy and commercialization of the expected growth can be attributed to the collaboration of several academic bodies and pharmaceutical companies, facilitating affordability before medicine development, and adoption and accessibility during clinical guidance.
Key Stages in the Value Chain
- Research & Development Leadership: Able to support continuous life-cycle innovation of ER therapies or patient-specific metabolic solutions.
- Regulated Manufacturers: Urging their partners to follow GMP-guided practices to produce and be compliant under a global leader's standardization and safety guidelines, such as Sobi, Pfizer, and/or small-molecule-manufactured brands.
- Distribution Manufacturing & Patient Engagement: Education could be reinforced to clarify the roles of liaisons and specialty logistics companies. A coordination effort between the rare disease network and hospital supply, as indicated within the cold-chain protocols that are managed post-deployment for delivery within hospitalization or the RA patient-assistance program (PAP).
Alkaptonuria Therapeutics Market Companies

- Bristol Myers Squibb
- Cipla
- Dr. Reddy's Laboratories Ltd.
- Eton Pharmaceuticals
- GSK plc
- Johnson & Johnson Services, Inc.
- Mallinckrodt Pharmaceuticals
- Pfizer Inc.
- Sanofi
- Sun Pharmaceutical Industries Ltd.
- Swedish Orphan Biovitrum AB
- Teva Pharmaceutical Industries Ltd.
Latest Announcements
- February 2025 – WashU Medicine
- Patricia Dickson, Centennial Professor of Pediatrics, Genetics Professor at WashU Medicine, and Director of The New Center.
- Announcement - Washington University School of Medicine in St. Louis has established the Center for Rare, Undiagnosed, and Genetic Diseases, supported by an USD 8.5 million grant from the Children's Discovery Institute (CDI). This initiative is a collaboration with St. Louis Children's Hospital, the St. Louis Children's Hospital Foundation, and WashU Medicine. The new center unites WashU Medicine researchers with the rare disease patient community, creating a collaborative network aimed at driving innovative research and accelerating drug discovery.
Recent Developments
- In September 2024, the Central Government launched a scheme aimed at developing 12 indigenous drugs to treat eight rare diseases. The program seeks to provide financial relief to many patients suffering from rare diseases in the country.
- In January 2024, Cycle Pharmaceuticals Ltd (Cycle) and Inceptua Group (Inceptua) announced a partnership to make NITYR (nitisinone) tablets available through a Free Goods Program for eligible patients with Hereditary Tyrosinemia Type 1 (HT-1) and Alkaptonuria (AKU).
Segments Covered in the Report
By Drug Type
- Dietary Supplements
- Nitisinone (p-hydroxyphenyl-pyruvate dioxygenase inhibitor)
- Pain Relief Medication
By Route of Administration
- Oral
- Parenteral
- Topical
By Patient Age Group
- Adult
- Geriatric
- Pediatric
By Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting