What is the Automated And Closed Cell Therapy Processing System Market Size?
The global automated and closed cell therapy processing system market size is accounted at USD 1.86 billion in 2025 and predicted to increase from USD 2.30 billion in 2026 to approximately USD 14.46 billion by 2035, representing a CAGR of 22.76% from 2026 to 2035. The growing demand for regenerative medicines is considered to fuel the growth of the global automated and closed-cell therapy processing system market.
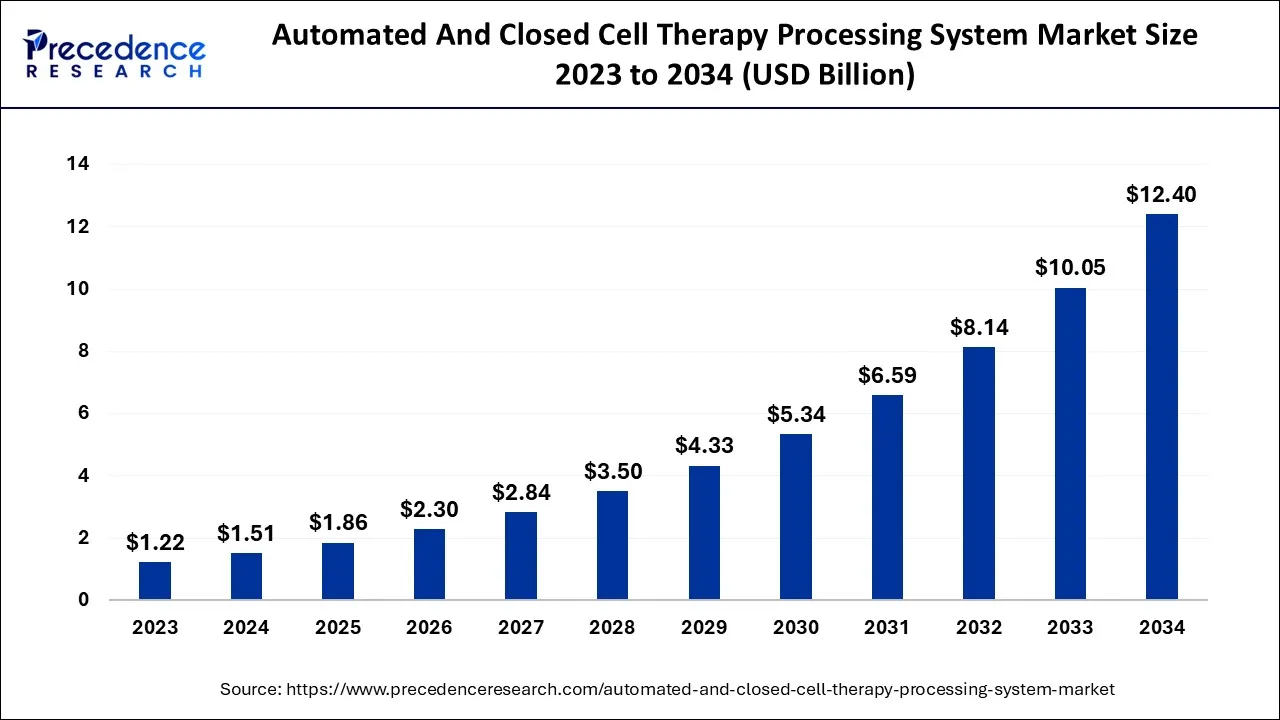
Automated And Closed Cell Therapy Processing System Market Key Takeaways
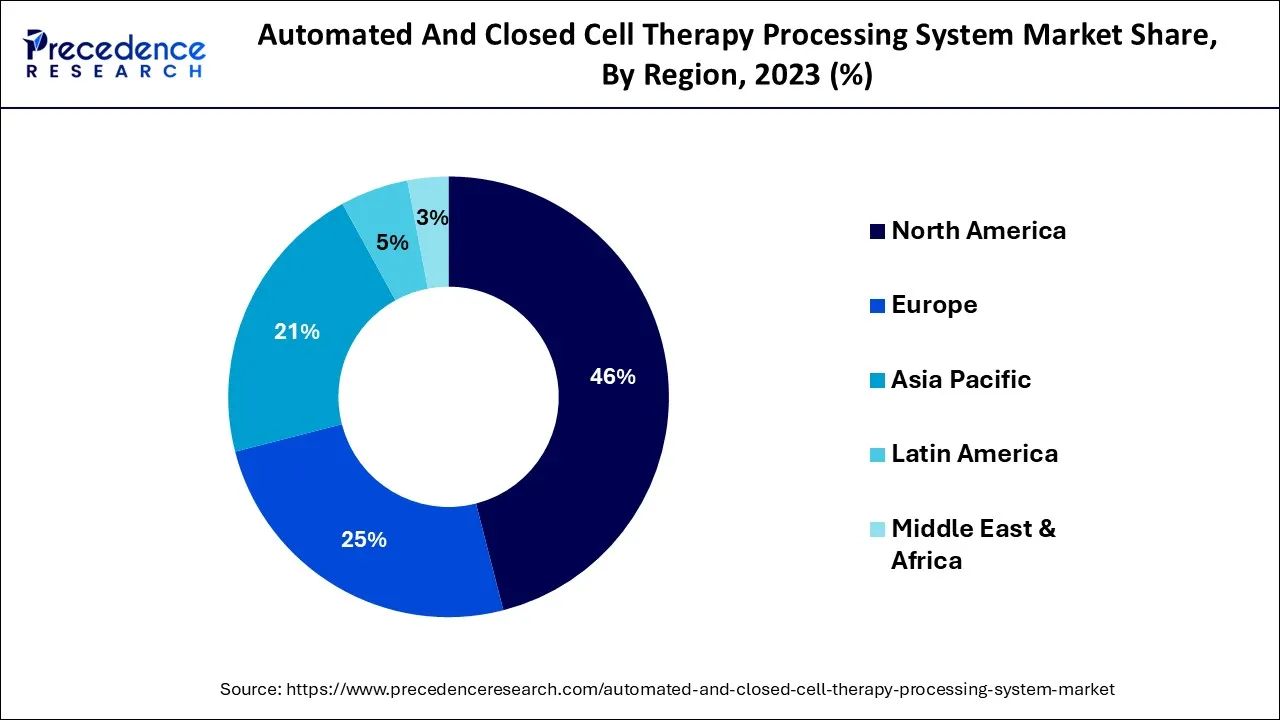
- North America generated more than 46% of the revenue share in 2025.
- By workflow, the expansion segment dominates the market and captures more than 35% of the revenue share in 2025.
- By workflow, the apheresis segment is projected to witness remarkable growth between 2026 and 2035.
- By type, the non-stem cell therapy segment dominates the market and generated more than 60% of the revenue share in 2025.
- By scale, The Research and development (R&D) scale segment captured more than 70% of the revenue share in 2025.
- By scale, the commercial scale segment is expected to register remarkable growth from 2026 to 2035.
Strategic Overview of the Global Automated and Closed Cell Therapy Processing System Industry
Automation in cell therapy processing or manufacturing systems has offered unmatched advantages to the global pharmaceutical industry. The cell therapy manufacturing process includes the selection, isolation, modification and expansion of the cells. Any minor error in this whole complex procedure may cause improper infusion of cells. The automated and closed cell therapy processing systems de-risk the final formulation. Automation in cell therapy processing reduces the chances of contamination led by the manual procedure. The automated and closed cell therapy processing system is used to enhance the scale of cell operations by reducing the risk of errors during bioprocessing.
The rising prevalence of cancer across the globe has increased the demand for automated and closed-cell therapy processing systems. The fully automated cell therapy processing systems aim to achieve robust processing with improved therapies for highly untreatable diseases. The prominent companies in the global automated and closed cell therapy processing systems market are supplementing the market's development by introducing advanced platforms for the cell therapy manufacturing process.
Artificial Intelligence: The Next Growth Catalyst in Automated and Closed Cell Therapy Processing Systems
AI is revolutionizing the automated and closed cell therapy processing system industry by shifting manufacturing from static, manual workflows to data-driven, intelligent ecosystems. Machine learning algorithms now provide real-time monitoring and predictive analytics to self-optimize culture conditions, such as pH and gas levels, within bioreactors, significantly reducing batch variability and failure rates.
AI-driven digital twins simulate bioprocessing conditions before physical runs, allowing manufacturers to optimize "vein-to-vein" delivery and scale production more efficiently. Furthermore, the integration of computer vision enables automated, non-invasive assessment of cell health and morphology, which streamlines quality control and ensures consistent therapeutic potency.
Automated And Closed Cell Therapy Processing System Market Growth Factors
The global automated and closed cell therapy processing systems market is expected to witness a significant increase during the projected timeframe owing to the continuously developing global pharmaceutical industry. The revolutionization of gene therapy and cell therapy has supplemented the development of the market in recent years. The rising commercialization of cell therapies around the world is considered to boost the market's growth in the upcoming years.
The utilization of automated and closed cell therapy processing systems has effectively reduced labor cost, this factor is also considered for offering profitable advantages to the market. At the same time, the development of the market is propelled by factors such as the rising demand for regenerative medicines and customized therapies across the globe.
The automated and closed cell therapy processing systems have offered the enhanced speed of manufacturing with high-quality outputs; this adventitious factor is fueling the market's development. Moreover, the Covid-19 pandemic has highlighted the advantages of stem cell therapy; the advancements in stem cell therapies are expected to continue during the forecast period by boosting the growth of the automated and closed cell therapy processing system market. However, the time-consuming and expensive cell therapy manufacturing process is considered to restrain the market's growth.
Market Outlook
- Market Growth Overview: The automated and closed cell therapy processing system market is expected to grow significantly between 2025 and 2034, driven by the need for process standardization & safety, integration of cutting-edge technology, and focus on cost reduction and scalability.
- Sustainability Trends: Sustainability trends involve transition to sustainable single-use technology, energy-efficient cleanroom in a box model, green logistics, and decentralized production.
- Major Investors: Major investors in the market include Danaher Corporation, Merck KGaA, Thermo Fisher Scientific Inc., OrbiMed, and Lonza Group AG.
- Startup Economy: The startup economy is focused on modular robotic platforms, point-of-care solutions, and high-throughput factories in a box.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 1.86 Billion |
| Market Size by 2035 | USD 14.46 Billion |
| Growth Rate from 2026 to 2035 | CAGR of 22.76% |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | By Workflow, By Type, and By Scale |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Driver
Rising demand for regenerative medicine
Regenerative medicine is a field of medicine that replaces damaged tissues or organs. Basically, regenerative medicine deals with replacing and restoring tissue or organ damage due to a particular disease or accident. The increasing demand for regenerative medicine from the healthcare sector is a significant driving factor for the growth of automated and closed-cell therapy processing systems, owing to the rising importance of stem cells in regenerative medicine.
The increasing cases of cancer and other chronic diseases that require prolonged treatment have boosted the popularity of regenerative medicine. Regenerative medicine consists of stem cell applications. Stem cells have indefinite cell division potential and are used as a frontline source for regenerative medicine.
Restraint
Shortage of raw materials:
The entire cell therapy manufacturing procedure consists of various steps which require starting material. The rising demand for automated and closed cell therapy processing systems across the globe has created a shortage of raw materials that are necessary for good medical practice (GMP). Due to the rapid increase in cell therapies on the note of rising chronic diseases, the market is facing a bottleneck in the availability of plasmids and viral vectors; they are proven GMP-based raw materials required in the cell therapy process.
Along with this, the obstacles in obtaining starting materials such as bone marrow, blood, and apheresis create a challenge for the market's growth. Thus, the shortage of raw materials is restraining the global automated and closed-cell therapy processing systems market.
Opportunity
Rising research and development activities in the pharmaceutical sector
Governments and major companies involved in the pharmaceutical industry are investing in research and development activities to enhance the discovery of novel treatments for various health conditions. The rising investment in R&D activities for the discovery of cell therapies will support the market's growth by facilitating the discovery of novel automated and closed-cell therapies. Along with this, the rising research and development activities in the pharmaceutical sector are considered to generate noticeable revenue for the market.
Workflow Insights
The expansion segment dominates the market and generated more than 35% of the revenue share in 2025. Expansion is observed as one of the most critical stages during cell therapy manufacturing. The segment aims at genetic modification, which plays a crucial role in the research process.
At the same time, the apheresis segment is expected to witness a significant increase during the forecast period. The apheresis segment includes the separation of different components from the donor's blood. The rising focus on blood management solutions during cell therapy processing is boosting the segment's growth. Apheresis offers better quality control of the components involved in automated cell therapy processing. However, the high cost of installing apheresis equipment may hinder the segment's growth.
Type Insights
Non-stem cell therapy dominates the global automated and closed cell therapy processing systems market. The segment dominates with a revenue share of over 60% and is expected to maintain growth during the forecast period. The rising focus on developing non-stem cell-based treatments for curative purposes is considered to boost the segment's growth. At the same time, the increasing prevalence of chronic diseases and neurological conditions is observed to promote the development of stem cell therapy during the forecast period.
Scale Insights
The Research and development (R&D) scale segment acquires almost 70% of the share in the global automated and closed cell therapy processing systems market. The rising number of innovation activities is supporting the segment's growth. At the same time, the increasing government investment in R&D activities to enhance the discovery of novel processing systems is observed to fuel the development of the R&D scale segment in the market. The growing number of clinical trials worldwide is one of the major factors boosting the growth of the R&D scale segment.
The commercial scale segment is projected to register significant growth during the forecast period due to rising companies' focus on the development of advanced cell therapies to be utilized in cancer treatment.
Regional Insights
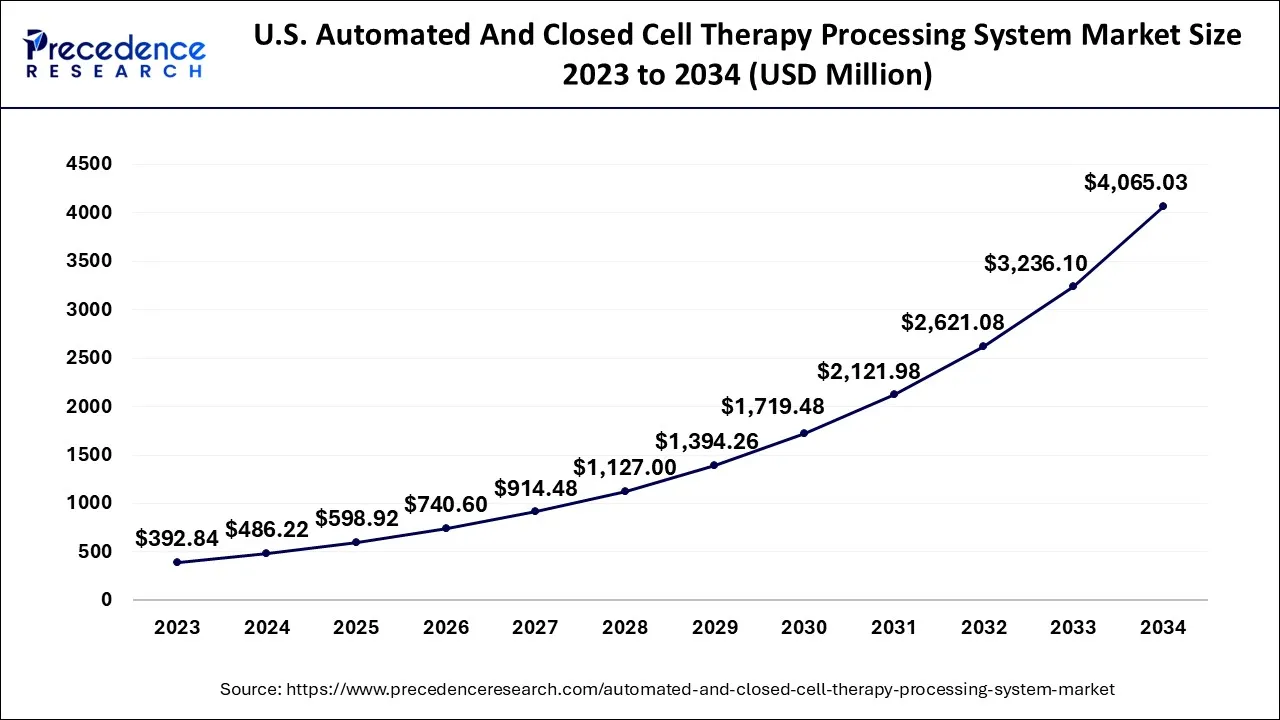
U.S. Automated And Closed Cell Therapy Processing System Market Size and Growth 2026 to 2035
The U.S. automated and closed cell therapy processing system market size is exhibited at USD 598.92 million in 2024 and is projected to be worth around USD 4,494.30 million by 2035, growing at a CAGR of 22.33% from 2026 to 2035.
North America accounts for the dominating share of almost 46%in the global automated and closed cell therapy processing systems market. The rapid adoption of advanced automated cell therapy manufacturing systems in the region is considered a significant factor for the dominating share. Along with this, the growing number of research institutes and investments for the same are fueling the market's growth in North America. The presence of major key players in the market has contributed to the advancements in the automated and closed cell therapy processing systems market.
The automated and closed cell therapy processing systems market in the United Kingdom is projected to grow at a CAGR of 21.2% during the forecast period.The well-established pharmaceuticals market and rising research and development activities in the biotech sector are observed to support the development of the market in Europe. At the same time, the rising demand for customized medicines in the region will help the market to grow at a noticeable rate.
Asia Pacific is expected to register a noticeable share in the automated and closed cell therapy processing systems market during the forecast period. A considerable potential of the pharmaceutical industry in India, Japan, China and South Korea is propelling the market's growth in Asia Pacific. The rich potential in Japan for the automation of cell therapy manufacturing systems will supplement the market's growth for Asia Pacific. Moreover, the advancements in the medicine development sector will fuel the growth of the automated and closed cell therapy processing systems market in Asia Pacific.
The automated and closed cell therapy processing systems market in Latin America, the Middle East and Africa is witnessing steady growth owing to therapeutics platforms or systems development. Moreover, the rising demand for regenerative medicines is observed to boost the market's growth during the forecast period.
U.S. Automated and Closed Cell Therapy Processing System Market Trends
The U.S. is rapidly transitioning from R&D to commercial-scale production to support a growing portfolio of FDA-approved treatments. The shift toward fully automated, closed systems is essential for meeting strict GMP standards while reducing contamination risks and human error. Integration of AI and digital twins is further optimizing "vein-to-vein" workflows through real-time monitoring and predictive quality control.
Additionally, the rise of decentralized, point-of-care manufacturing is localizing production to hospitals, significantly improving patient turnaround times.
China Automated and Closed Cell Therapy Processing System Market Trend
China's robust government support and a strong pipeline of innovative clinical trials for therapies targeting hematologic malignancies and solid tumors. The primary trend is the rapid adoption of integrated automation platforms, such as those from Thermo Fisher and Miltenyi Biotec, to standardize manufacturing processes for scalability and reduce reliance on manual labor. Domestic innovation is increasing, with local players like Cellular Biomedicine Group accelerating R&D to meet local regulatory standards (NMPA) for commercialization.
How Did Europe Notably Grow in the Automated and Closed Cell Therapy Processing System Market?
Europe streamlined multi-country clinical trials and biomanufacturing. Strategic public-private funding, including the EASYGEN Consortium and Germany's Biotech Strategy, accelerated the adoption of automated, closed-system bioreactors. The region pivoted to industrial-scale production using modular "cleanroom-in-a-box" systems and AI-powered quality control to meet the massive projected demand for CAR-T therapies.
Germany Automated and Closed Cell Therapy Processing System Market Trend
Germaine's integration of AI and digital twins, evidenced by new platforms, has introduced unprecedented precision in real-time monitoring and quality assurance. Furthermore, the industry is rapidly shifting toward commercial-scale operations to accommodate the increasing volume of approved cell therapies. Consequently, Germany's focus on automation and digitalization is creating a highly scalable and cost-effective infrastructure for the future of personalized medicine.
Top Companies in the Automated and Closed Cell Therapy Processing System Market & Their Offerings:
- Lonza
Lonza provides the Cocoon Platform, a fully automated and closed "patient-in-a-box" system designed to streamline the manufacturing of personalized cell therapies. - BioSpherix
BioSpherix specializes in the Cytocentric platform, which provides fully closed, aseptic environments that precisely mimic physiological conditions for cell processing. - Cellares
Cellares developed the Cell Shuttle™, a high-throughput automated factory-in-a-box capable of manufacturing up to 16 patient doses simultaneously. Their platform addresses scalability challenges by automating the entire end-to-end process, significantly reducing costs and "vein-to-vein" time for CAR-T therapies. - Sartorius
Sartorius offers a comprehensive portfolio of automated bioreactors and specialized single-use technologies, such as the Ambr and BIOSTAT systems, for cell expansion and harvesting. - Cytiva
Cytiva provides a robust suite of automated solutions, including the Sefia™ and Xuri™ platforms, which standardize cell isolation, expansion, and harvesting in a closed environment. - ThermoGenesis Holdings Inc.
ThermoGenesis offers the CAR-TXpress™ platform, a suite of automated, closed systems specifically designed to improve the efficiency of cell processing and cryopreservation. Their proprietary buoyancy-activated cell sorting (BACS) technology provides high-recovery cell isolation, which is critical for the initial stages of cellular therapy manufacturing. - Thermo Fisher Scientific Inc.
Thermo Fisher provides an end-to-end ecosystem of automated systems, such as the Gibco™ CTS™ DynaCellect™ and Rotea™ platforms, for precise cell isolation and washing.
Automated and Closed Cell Therapy Processing System Market Companies
- Lonza
- BioSpherix
- Cellares
- Sortorius
- Cytiva
- ThermoGenesis Holdings Inc
- Thermo Fisher Scientific Inc
Recent Developments
- In February 2023,a clinical-stage biopharmaceutical company that develops innovative cell therapies, IASO Biotherapeutics, announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track (FT) Designation and Regenerative Medicine Advanced Therapy (RMAT) Designation to its new BCMA CAR-T CT103A (Equecabtagene Autoleucel) drug. The drug can be utilized in the treatment of relapsed/refractory multiple myeloma (RRMM), which is a severe type of blood cancer that becomes non-responsive to cell therapy.
- In February 2023,an independent organization for innovation and technology in the UK,Cell and Gene TherapyCatapult, announced its involvement in the development of a significant new life science campus in Stevenage. The company has planned to invest approximately 900 million Euros in the development of the new campus. The new campus aims to meet the enormous demand from life sciences companies.
- In February 2023,a prominent cell therapy and regenerative medicine developer, Athersys Inc., announced that the U.S. Food and Drug Administration (FDA) granted a clinical type B meeting for protocol discussion. This step is intended to boost the company's capabilities in the clinical trials for the advanced MASTERS-2.
- In April 2022,one of the most extensive cancer research and treatment organizations in the U.S., City of Hope, planned to integrate Curate Biosciences's Curate Cell Processing System into its workflow in order to manufacture investigational CAR-T cell immunotherapy. The company believes in achieving an innovative approach to T-cell separation by integrating this cell processing system.
- In May 2021,a leading developer of cell therapies, GPB Scientific, announced adding $18 million in financial support for the commercialization of the Curate Cell Processing System by Curate Biosciences. This new generation cell processing system is expected to enable optimized development in the CAR-T and TCR programs. The Curate system also utilized the GPB's Proprietary Deterministic Cell Separation Technology to deliver unmatched gene therapies for cancer treatment.
- In March 2025, Limula launched LimONE, an automated, closed, single-use system designed to standardize and scale CAR-T and gene-edited cell therapy manufacturing. LimONE enables end-to-end production of highly personalized treatment and reduction of labour, infrastructure, and equipment cots. (https://limula.ch)
- In February 2025, Cellistic introduced Allo Chassis, ready-to-use, immune-cloaked iPSC lines that provide a standardized starting material for scalable, "off-the-shelf" (allogeneic) cell therapies. Allo Chassis represents a sustainable leap toward cell line development. (https://bioinformant.com)
Segments Covered in the Report
By Workflow
- Cryopreservation
- Fill-finish
- Separation
- Expansion
- Apheresis
- Others
By Type
- Stem Cell Therapy
- Non-stem Cell Therapy
By Scale
- Commercial Scale
- R&D Scale
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting