Ceftazidime-Avibactam Market Size and Forecast 2025 to 2034
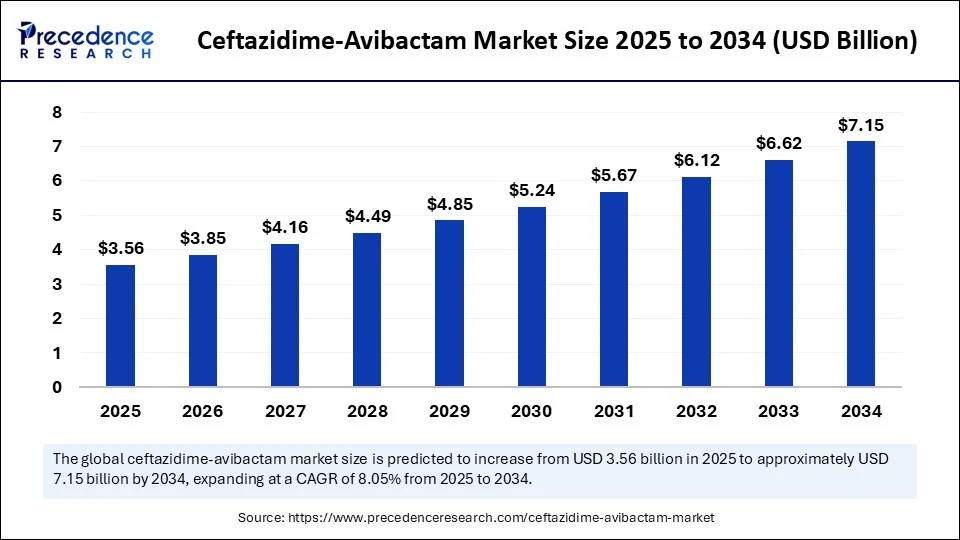
The global ceftazidime-avibactam market size accounted for USD 3.3 billion in 2024 and is predicted to increase from USD 3.56 billion in 2025 to approximately USD 7.15 billion by 2034, expanding at a CAGR of 8.05% from 2025 to 2034. The market for ceftazidime-avibactam is experiencing substantial growth due to the rising incidence of multidrug-resistant gram-negative infections, which is driving its demand in clinical settings. This demand is further reinforced by its effectiveness in treating complicated intra-abdominal and urinary tract infections. Increasing hospital utilization and heightened awareness of antimicrobial resistance are expected to accelerate market expansion.
Ceftazidime-Avibactam MarketKey Takeaways
- In terms of revenue, the global ceftazidime-avibactam market was valued at USD 3.3 billion in 2024.
- It is projected to reach USD 7.15 billion by 2034.
- The market is expected to grow at a CAGR of 8.05% from 2025 to 2034.
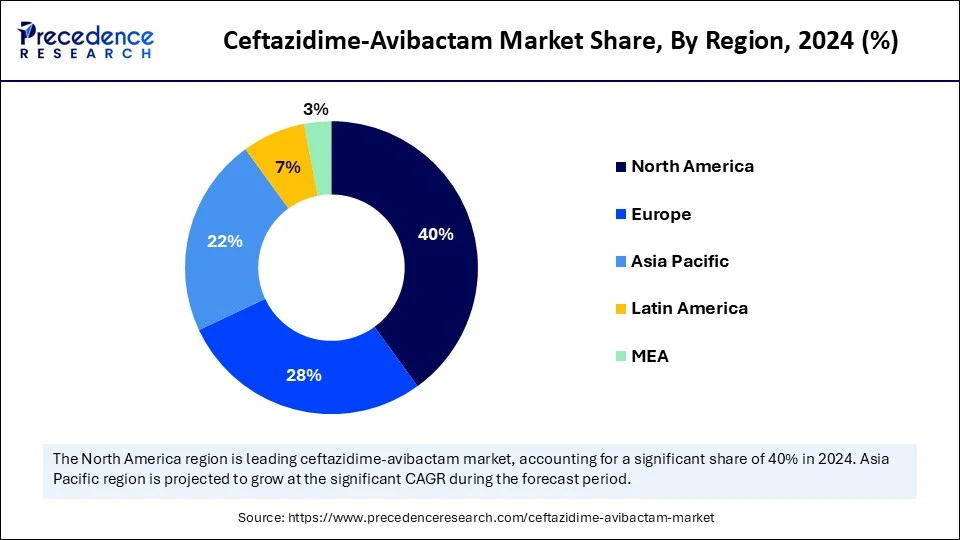
- North America dominated the global ceftazidime-avibactam market with the largest share of 40% in 2024.
- Asia Pacific is anticipated to grow at a significant CAGR from 2025 to 2034.
- By formulation, the intravenous (IV) injection segment captured the biggest market share 65% in 2024.
- By formulation, the lyophilized powder segment is anticipated to grow at a significant CAGR from 2025 to 2034.
- By indication, the complicated urinary tract infections (cUTIs) segment contributed the highest market share of 35% in 2024.
- By indication, the hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) segment is expected to witness the fastest CAGR during theforeseeable period
- By end user, the hospitals segment held the major market share of 70% in 2024.
- By end user, the ambulatory care centers segment is projected to grow at a notable CAGR between 2025 and 2034.
- By distribution channel, the hospital pharmacy segment captured the largest market share of 60% in 2024.
- By distribution channel, the online pharmacy segment is anticipated to grow at a significant CAGR from 2025 to 2034.
How Can AI Impact the Ceftazidime-Avibactam Market?
Artificial intelligence (AI) is transforming the ceftazidime-avibactam market, especially in resistance detection, treatment optimization, and drug development. AI can analyze data from MALDI-TOF MS (Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry) to quickly identify resistance to ceftazidime-avibactam, particularly in Klebsiella pneumoniae. This rapid detection allows for timely adjustments in treatment plans. AI is also used to screen large datasets of chemical compounds to find potential new antibiotics. By improving the speed and accuracy of resistance detection, slow the spread of antimicrobial resistance, a vital aspect of public health.
U.S. Ceftazidime-Avibactam Market Size and Growth 2025 to 2034
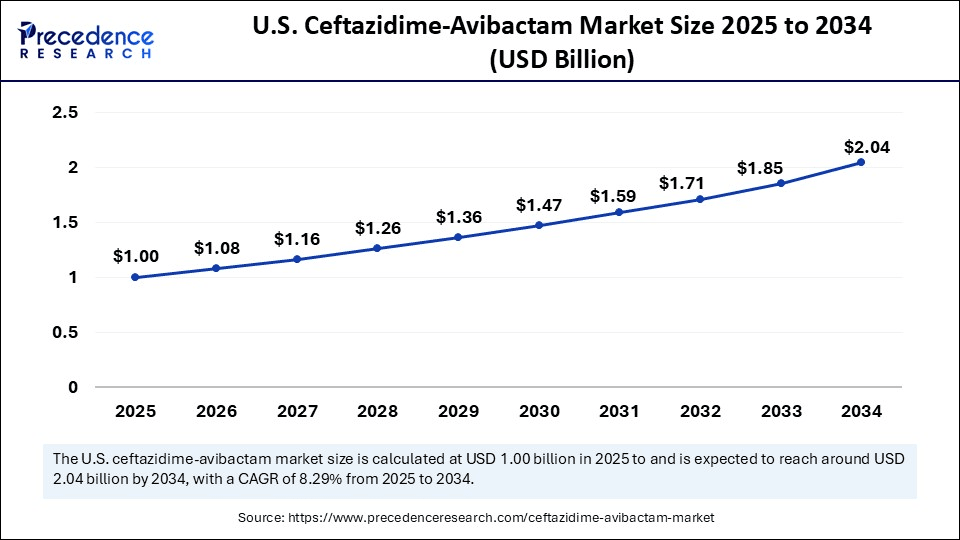
The U.S. ceftazidime-avibactam market size was exhibited at USD 0.92 billion in 2024 and is projected to be worth around USD 2.04 billion by 2034, growing at a CAGR of 8.29% from 2025 to 2034.
How did North America lead the ceftazidime-avibactam market in 2024?
North America dominated the ceftazidime-avibactam market in 2024, largely due to the high incidence of MDR gram-negative infections, a favorable regulatory environment, and robust antimicrobial stewardship programs. The region's strong healthcare infrastructure, combined with a well-developed R&D ecosystem, fosters innovation in antimicrobial therapies. Advanced diagnostic tools and rapid testing methods enable targeted and efficient use of antibiotics like ceftazidime-avibactam, leading to improved patient outcomes and reduced development of resistance. Prominent players such as Allergan and Pfizer contribute to this dynamic. Additionally, regulatory bodies like the U.S. FDA have implemented initiatives such as the Generating Antibiotic Incentives Now (GAIN) Act to incentivize and expedite the development and approval of new antibiotics, including ceftazidime-avibactam.
- In February 2025, AbbVie announced that the U.S. Food and Drug Administration approved EMBLAVEO™ as the first and only fixed-dose intravenous monobactam/β-lactamase inhibitor combination antibiotic. This product is approved in combination with metronidazole for patients 18 years and older who have limited or no alternative options for treating cIAI. (Source: https://news.abbvie.com)
The U.S. ceftazidime-avibactam market trends
The U.S. significantly influences the global market due in large part to early FDA approval, which facilitates the global introduction and adoption of this antibiotic. The U.S. holds a substantial market share, generating significant sales of new anti-CRE antibiotics, including CAZ-AVI. The country fosters clinical research and development, enhancing the understanding and application of ceftazidime-avibactam. However, the U.S. also faces challenges related to antimicrobial resistance, necessitating diligent stewardship to ensure the long-term efficacy of ceftazidime-avibactam. Rising generic alternatives and the potential for overuse pose risks for increasing resistance.
Canada ceftazidime-avibactam market trends
Canada plays an evolving role in the global market, emphasizing antimicrobial stewardship to guide appropriate antibiotic prescribing and minimize resistance development. Canada's participation in international initiatives like CARB-X and its investments in vaccine and therapeutic development, along with efforts to improve surveillance, reflect a commitment to addressing antimicrobial resistance (AMR) broadly. This positions Canada to positively influence the market through the promotion of responsible use and ongoing research into effective treatment options.
How will Asia Pacific become the fastest-growing region in the ceftazidime-avibactam market?
Asia-Pacific holds the fastest-growing market for ceftazidime-avibactam. This growth is primarily driven by the increasing prevalence of infectious diseases and antimicrobial resistance, expanding healthcare infrastructure, rising healthcare spending, and growing awareness of advanced therapies. Countries such as India and China experience a high prevalence of infections and increasing rates of antimicrobial resistance, particularly to carbapenems. The growing recognition of the need for effective treatments for severe infections drives the adoption of innovative drugs like ceftazidime-avibactam.
Market Overview
Ceftazidime-avibactam is a combination antibiotic specifically used to treat severe Gram-negative bacterial infections, particularly those caused by multidrug-resistant organisms, such as Enterobacteriaceae and Pseudomonas aeruginosa. It is commonly administered in hospital environments for complicated urinary tract infections (cUTIs), complicated intra-abdominal infections (cIAIs), and hospital-acquired pneumonia (HAP). This combination allows ceftazidime to effectively target a broader range of bacteria, including strains that have developed resistance to other antibiotics. The market for this drug is driven by the increasing prevalence of antibiotic-resistant bacteria, the drug's effectiveness against these resistant strains, and its approval for treating various types of infections.
What are the Key Trends in the Ceftazidime-Avibactam Market?
- Effectiveness against resistant strains: eftazidime-avibactam has demonstrated both in vitro and in vivo activity against numerous multidrug-resistant (MDR) Gram-negative bacteria, including those producing extended-spectrum beta-lactamases (ESBLs) and carbapenemases.
- Growing awareness and adoption: There is increasing awareness among healthcare professionals regarding the availability and efficacy of ceftazidime-avibactam, along with its incorporation into treatment protocols, which is contributing to market expansion.
- Favorable pharmacokinetic and pharmacodynamic properties: Ceftazidime-avibactam boasts a favorable pharmacokinetic and pharmacodynamic profile, making it effective in treating infections while ensuring safe administration, further promoting its acceptance.
- Focus on antibiotic stewardship: Efforts to optimize antibiotic use and combat resistance, including stewardship programs, can lead to more appropriate and targeted use of ceftazidime-avibactam, potentially enhancing its market growth.
- Clinical trials and research: Current clinical trials and research demonstrating the efficacy and safety of ceftazidime-avibactam in treating infections caused by resistant bacteria continue to underpin market expansion.
- Regulatory approvals: The approvals from the FDA and EMA for ceftazidime-avibactam for specific indications have stimulated market growth by increasing access and availability.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 7.15 Billion |
| Market Size in 2025 | USD 3.56 Billion |
| Market Size in 2024 | USD 3.3 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.05% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Formulation, Indication, End User, Application, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising prevalence of multidrug-resistant (MDR) bacteria
The primary driver in the ceftazidime-avibactam market is the rising prevalence of multidrug-resistant (MDR) bacteria, particularly carbapenem-resistant Enterobacteriaceae (CRE) and Pseudomonas aeruginosa. These superbugs pose a significant threat to global public health, making ceftazidime-avibactam a crucial therapeutic option due to its effectiveness against such resistant pathogens. It is frequently used to treat cUTIs, cIAIs, and HAP. This resistance necessitates the development of new treatment options, such as ceftazidime-avibactam, which expands the activity spectrum of ceftazidime to include many resistant strains.
Restraint
The potential for indiscriminate or empirical use
A significant concern in this market is the potential for indiscriminate or empirical use, particularly as the drug loses exclusivity and becomes available as a generic in countries like India. Ceftazidime-avibactam is a last-line antibiotic for targeted therapy of specific carbapenem-resistant Gram-negative infections. Uncontrolled access and aggressive marketing of generic versions, especially at lower prices, could lead to overuse. Such overuse, particularly in regions with weak antibiotic stewardship programs (like India), could promote the development and spread of resistance to this and other critical antibiotics.
Opportunity
Expanding its use as a targeted therapy
Looking ahead, a key opportunity in the ceftazidime-avibactam market lies in expanding its use as a targeted therapy against a broader range of multidrug-resistant gram-negative infections, especially in countries with a high prevalence of specific resistance mechanisms. This antibiotic is already valuable for treating serious infections caused by resistant bacteria, including carbapenem-resistant Enterobacterales and Pseudomonas aeruginosa. Furthermore, when used in combination with Aztreonam, ceftazidime-avibactam offers a treatment option for infections caused by MDR Gram-negative bacteria that produce various β-lactamases, including MBLs and serine carbapenemases.
Formulation insights
What made the intravenous (IV) injection segment lead the ceftazidime-avibactam market in 2024?
The intravenous (IV) injection segment led the market in 2024. This is due to the nature of the infections it treats and the pharmacokinetic properties of the drug. Ceftazidime-avibactam is indicated for serious, complicated infections like cIAI, cUTI, HAP, and ventilator-associated bacterial pneumonia (VABP). These infections often require immediate and robust antimicrobial treatment to ensure optimal efficacy when drug concentrations stay above the minimum inhibitory concentration (MIC) for a prolonged period. IV administration, especially via prolonged infusion, provides more consistent and sustained drug levels, maximizing the effectiveness of this combination against resistant pathogens and driving the demand for quickly administered and rapidly acting formulations like IV injections.
The lyophilized powder formulation is expected to experience the fastest growth during the forecast period. This is mainly due to the benefits of lyophilization for parenteral drugs, which help ensure the drug remains potent and available when needed. Lyophilization removes moisture from the drug, making it more stable and less prone to degradation. This is especially important for antibiotics like ceftazidime-avibactam, which are often sensitive to temperature and moisture, allowing for extended shelf life and improved long-term storage and transportation. It is a preferred technique for many biologics and parenteral pharmaceuticals, including antibodies and vaccines, to maintain their potency efficiently and cost-effectively.
Indication insights
How did the complicated urinary tract infections (cUTIs) segment lead the ceftazidime-avibactam market in 2024?
The complicated urinary tract infections (cUTIs) segment led the market in 2024. This is mainly due to its broad-spectrum activity and the increasing prevalence of MDR Gram-negative bacteria, particularly Extended-Spectrum Beta-Lactamase (ESBL)-producing Enterobacterales and Carbapenem-Resistant Enterobacterales (CRE), which have made treating cUTIs more challenging. Ceftazidime-avibactam is effective against many of these resistant strains, providing a crucial treatment option when other antibiotics have failed. Its broader spectrum makes it valuable for empiric or targeted therapy in cUTIs, addressing unmet clinical needs and achieving positive outcomes.
The hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) segments are expected to grow the fastest. This growth is mainly due to the rising prevalence of antibiotic-resistant Gram-negative bacteria causing these infections, combined with ceftazidime-avibactam's proven efficacy against resistant strains. The increasing resistance calls for more effective treatment options, making ceftazidime-avibactam a key choice for HAP and VAP. Additionally, rising awareness among healthcare providers about its effectiveness in these cases and the growing threat of resistance are driving its adoption.
End user Insights
Why did the hospitals segment lead the ceftazidime-avibactam market in 2024?
The hospitals segment dominated the market in 2024. This is primarily because of the drug's effectiveness in treating serious, hospital-acquired infections caused by MDR Gram-negative bacteria. Hospitals are the primary sites for managing these complex infections, making them the main end-users. Ceftazidime-avibactam is often used as targeted therapy for resistant infections, further strengthening its role in hospitals. Hospitals also have antibiotic stewardship programs that guide appropriate use, ensuring it is prescribed only for eligible patients and preventing overuse. Much of the research on ceftazidime-avibactam occurs in hospital settings, further emphasizing its importance there.
The ambulatory care centers segment is experiencing the fastest growth during the foreseeable period. This is due to the rising prevalence of MDR infections and carbapenem-resistant organisms (CROs), which are shifting treatment toward outpatient care and oral formulations. Healthcare systems aim to reduce hospitalization costs and improve patient convenience and adherence by offering effective therapies in outpatient settings. Additionally, integrating precision medicine and rapid diagnostics within hospital and outpatient care helps quickly identify resistant strains, leading to better patient outcomes and lower healthcare costs.
Distribution channel insights
How did the hospital pharmacy segment dominate the ceftazidime-avibactam market in 2024?
The hospital pharmacy segment maintained a dominant position in the market. This is because of its direct connection with healthcare providers and specialized distribution channels. Its dominance is also driven by the demand for these antibiotics to treat serious, often hospital-acquired, infections caused by MDR bacteria. Pharmaceutical companies actively engage with hospital pharmacists and prescribers to educate them about proper dosing, side effects, and the role of ceftazidime-avibactam in fighting resistant infections. Hospital antimicrobial stewardship programs, usually led by pharmacists, are crucial in optimizing antibiotic use, including appropriate selection and administration of ceftazidime-avibactam.
The online pharmacy segment is also experiencing rapid growth, especially in distribution channels. This trend is fueled by increased internet and smartphone use, the convenience of home delivery, and the rise of telehealth and digital prescriptions. Online pharmacies enable patients to order medications from home and receive them conveniently, which is especially helpful for those with mobility issues or limited access to physical pharmacies. The widespread use of the internet and smartphones for healthcare-related tasks, along with telehealth integration, allows for remote consultations and digital prescriptions, further boosting growth by offering competitive pricing and discounts, attracting price-sensitive consumers.
Value Chain Analysis
- R&D
Research and development (R&D) efforts focus on combating rising antibiotic resistance by investigating resistance mechanisms and exploring combination therapies, particularly with aztreonam, to counteract the spread of generic versions and ensure long-term efficacy.
Key Players: Pfizer Inc., Allergan plc., AstraZeneca plc., Alkem Laboratories Ltd.
- Clinical Trials and Regulatory Approvals
It has undergone extensive clinical trials and received regulatory approvals in various regions, including the U.S. and Europe, for use in adults and children for conditions such as complicated intra-abdominal infections, complicated urinary tract infections, and hospital-acquired pneumonia.
Key Players: AbbVie, Intellicure Lifesciences, Natco Pharma Ltd., BDR Pharmaceuticals.
- Formulation and Final Dosage Preparation
Ceftazidime-avibactam is an IV antibiotic that treats serious Gram-negative bacterial infections, supplied as a powder requiring reconstitution and dilution before infusion. The market is driven by rising antimicrobial resistance, particularly from pathogens like CRE and P. aeruginosa, and is propelled by innovations in beta-lactamase inhibitors like avibactam.
Key Players: AstraZeneca, Pfizer, Intelicure Lifesciences, and Alkem Laboratories.
- Packaging and Serialization
Ceftazidime-avibactam is typically packaged in single-use glass vials, each containing 2g of Ceftazidime and 0.5g of Avibactam in sterile powder form. Serialization involves assigning a 2D barcode to each product unit and its packaging, which enables tracking, ensures authenticity, combats counterfeiting, and enhances patient safety.
Key Players: Gerresheimer AG, SCHOTT AG, Recipharm, Nipro Corporation, SGD Pharma.
- Distribution to Hospitals, Pharmacies
Distribution primarily targets hospitals and pharmacies, reflecting its crucial role as an antibiotic for serious infections. While its initial availability was limited to hospitals, the emergence of generics potentially expanded its presence in community pharmacies. This has raised concerns regarding antibiotic stewardship and appropriate usage.
Kay Players: AstraZeneca plc, Aark Pharmaceuticals, Natco Pharma Ltd., Pfizer Inc.
- Patient Support and Services
In addition, there is a focus on helping patients navigate the complexities of using this last-line antibiotic. This includes providing information and education on its use, potential side effects, dosage adjustments (especially in cases of kidney issues), and managing associated challenges such as antimicrobial resistance.
Key Players: Merck and Co., Wockhardt Ltd., Torso Healthcare, Teva Pharmaceutical Industries Ltd., Alkem Laboratories Ltd.
Ceftazidime-Avibactam Market Companies

- Pfizer Inc.
- Allergan plc
- Cipla Limited
- Sun Pharmaceutical Industries Ltd.
- Dr. Reddy's Laboratories
- Sandoz International GmbH
- Hikma Pharmaceuticals
- Aurobindo Pharma Ltd.
- Lupin Limited
- Teva Pharmaceutical Industries Ltd.
- Fresenius Kabi AG
- Mylan N.V.
- Zydus Cadila
- Torrent Pharmaceuticals Ltd.
- Amneal Pharmaceuticals
- Hetero Drugs Ltd.
- Glenmark Pharmaceuticals
- Natco Pharma Ltd.
- Jubilant Life Sciences
- Alembic Pharmaceuticals
Leaders Announcements
- In April 2024, Adalvo is progressing with the development of Ceftazidime + Avibactam, aiming for a Day-1 launch in all major markets. This antibiotic is designed for complicated intra-abdominal infections, complicated urinary tract infections, and hospital-acquired pneumonia, demonstrating the company's commitment to efficiently meeting patient demands for affordable medicine. (Source: https://www.adalvo.com)
Recent Developments
- In February 2023, Alkem launched a novel anti-infective, an MDR antibiotic, in India under the brand name Zidavi. This product combines ceftazidime and avibactam and is recommended by the Infectious Diseases Society of America and the Indian Council of Medical Research. Zidavi is an effective treatment for carbapenem-resistant infections, addressing the growing challenge of antibiotic resistance for treating infections complicated by MDR organisms.(Source: https://www.healthcareradius.in)
- In January 2023, BDR Pharmaceuticals introduced XAVITAZ, a generic combination of Ceftazidime and Avibactam, effective against resistant gram-negative infections. This product aims to improve access to treatment for serious conditions such as hospital-acquired pneumonia and intra-abdominal infections. Raheel Shah from BDR Group emphasized their commitment to providing quality treatment.(Source: https://www.expresspharma.in)
Segments Covered in the Report
By Formulation
- Intravenous (IV) Injection
- Lyophilized Powder
- Others
By Indication
- Complicated Urinary Tract Infections (cUTIs)
- Complicated Intra-Abdominal Infections (cIAIs)
- Hospital-Acquired Pneumonia (HAP) and Ventilator-Associated Pneumonia (VAP)
- Bloodstream Infections (BSIs)
- Others
By End User
- Hospitals
- Clinics
- Ambulatory Care Centers
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting