Immuno-Oncology Antibodies Market Size and Forecast 2025 to 2034
The immuno-oncology antibodies markets report analyzes key trends, market size, and major players shaping the future of cancer treatment. The market is experiencing significant growth due to the increasing prevalence of cancer and the shift toward targeted therapies. This market's growth is further fostered by advancements in monoclonal and bispecific antibodies, which enhance the immune system's response against tumors. Additionally, the rise in clinical trials and approvals for novel antibodies is expected to accelerate market expansion in the coming years.
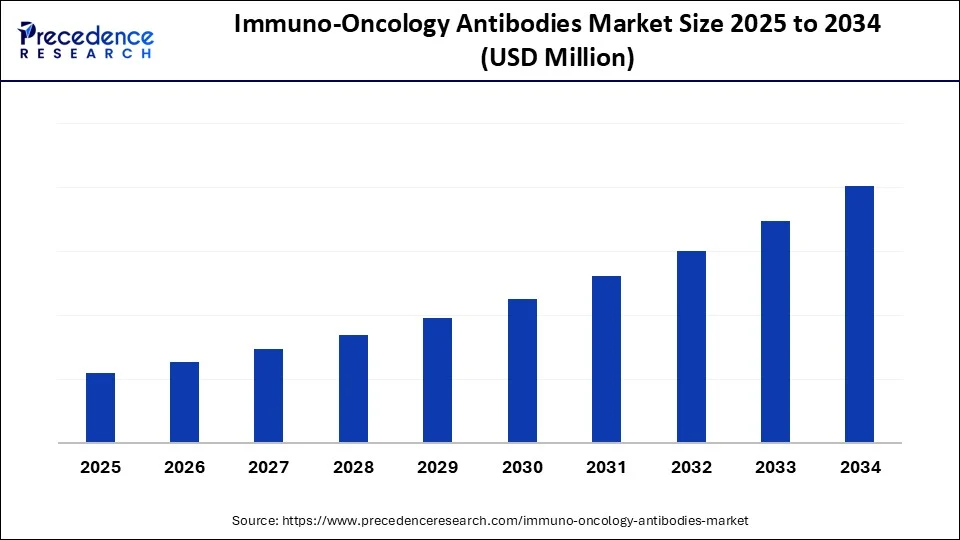
Immuno-Oncology Antibodies Market Key Takeaways
- North America dominated the immuno-oncology antibodies market with the largest share of 48.7% in 2024.
- Asia Pacific is expected to grow at the fastest CAGR from 2025 to 2034.
- By drug class, the checkpoint inhibitors segment led the market in 2024, under which the PD-1 inhibitors hold a 32.1% as a sub-segment.
- By drug class, the bispecific antibodies segment is expected to grow at the fastest CAGR in the upcoming period.
- By application, the non-small cell lung cancer (NSCLC) segment captured the biggest market share of 28.4% in 2024.
- By application, the triple-negative breast cancer (TNBC) segment is expected to grow at a significant CAGR over the projected period.
- By mechanism of action, the immune checkpoint blockade segment contributed the biggest market share of 45.7% in 2024.
- By mechanism of action, the immune cell redirection segment is anticipated to grow at a significant CAGR from 2025 to 2034.
- By route of administration, the intravenous (IV) segment held the highest market share of 81.3% in 2024.
- By route of administration, the subcutaneous (SC) segment is expanding at a significant CAGR from 2025 to 2034.
- By end user, the hospitals segment held the major market share of 61.2% in 2024.
- By end user, the cancer specialty clinics segment is projected to grow at a significant CAGR between 2025 and 2034.
- By distribution channel, the hospital pharmacies segment generated the largest market share of 52.6% in 2024.
- By distribution channel, the specialty pharmacies segment is expected to experience rapid growth during the forecast period.
How Can AI Impact the Immuno-Oncology Antibodies Market?
The use of artificial intelligence (AI) is revolutionizing the market by accelerating research, improving treatment strategies, and boosting drug development. AI tools help identify biomarkers, predict treatment responses, and refine antibody designs, leading to more personalized and effective cancer therapies. They analyze large datasets of genomic, proteomic, and imaging data to find new biomarkers that predict patient responses to immuno-oncology treatments. AI can forecast individual treatment responses, allowing clinicians to select the most appropriate therapies and optimize strategies, which can significantly impact market growth.
Market Overview
The immuno-oncology antibodies market refers to the global industry focused on therapeutic monoclonal antibodies (mAbs) and related antibody formats designed to harness the body's immune system to recognize, target, and eliminate cancer cells. These antibodies mainly work by modulating immune checkpoints (e.g., PD-1, PD-L1, CTLA-4), stimulating T-cell activation, redirecting immune cells (e.g., bispecific T-cell engagers), or killing tumor cells through antibody-dependent mechanisms. Their applications span multiple types of cancer, often as monotherapy or combined with chemotherapy, targeted therapy, or other immunotherapies. This market is growing rapidly due to rising cancer rates, advances in personalized medicine, and the development of targeted therapies and companion diagnostics.
What are the Key Trends in the Immuno-Oncology Antibodies Market?
- Advancements in Immunotherapy: Research and development in immuno-oncology have led to major breakthroughs, including immune checkpoint inhibitors like PD-1/PD-L1 inhibitors, CAR-T cell therapy, and bispecific antibodies.
- Clinical Pipeline:The extensive pipeline of immuno-oncology drugs, with many therapies in various stages of development and new immuno-oncology antibodies being investigated, indicates ongoing innovation and potential for future market expansion.
- Improved Patient Outcomes:Immuno-oncology therapies have demonstrated the ability to produce durable responses and improve survival rates in certain cancers. The identification and use of biomarkers to predict patient response to these therapies are further accelerating the development and adoption of immuno-oncology treatments.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Class, Mechanism of Action, Route of Administration, Application, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increasing Prevalence of Cancer and Technological Advances
The primary driver of the immuno-oncology antibodies market is the increasing prevalence of cancer worldwide. This, in turn, creates the urgent need for effective treatments, including immune-oncology therapies. Innovations such as checkpoint inhibitors, CAR-T cell therapy, and bispecific antibodies are driving significant advances in cancer treatment. Improvements in bioinformatics and genomics are enabling the creation of personalized immuno-oncology treatments tailored to individual patients. Immuno-oncology therapies are expanding beyond traditional indications, exploring applications in solid tumors, pediatric cancers, and combination treatment regimens. Supportive regulations and accelerated approval pathways by regulatory bodies for immunotherapy and personalized medicine also propel the growth of the market.
Restraint
Higher Cost of Therapies
The main challenge in this market is the high cost of therapies, which include development, manufacturing, and delivery expenses, making these treatments unaffordable or inaccessible for many patients and healthcare systems. The complex process of developing and producing monoclonal antibodies involves extensive research, clinical trials, and specialized manufacturing facilities, which contribute to their high costs. This restricts access, especially in low- and middle-income countries, and insurance disparities can also limit patient access even in wealthier nations. Moreover, the limited efficacy of immune-oncological therapies may restraint market growth.
Opportunity
Development of Novel Combination Therapies
A significant future opportunity for the immune-oncology antibodies market lies in developing novel combination therapies, particularly those targeting multiple pathways or combining immuno-oncology agents with other treatment modalities. This includes exploring new targets beyond PD-1/PD-L1 and CTLA-4 and integrating immuno-oncology with targeted therapies, chemotherapy, or radiation. Of particular interest are bispecific antibodies that can redirect T-cells and modulate immune checkpoints, as well as efforts to modify the tumor microenvironment, focusing on factors that promote immune suppression or resistance. Combining various immuno-oncology approaches or integrating them with other cancer treatments, such as chemotherapy, radiation, or targeted therapies, has shown promising results in clinical trials and is anticipated to boost market growth.
Drug Class Insights
What Made Checkpoint Inhibitors the Dominant Segment in the Immuno-Oncology Antibodies Market in 2024?
The checkpoint inhibitors segment dominated the market, under which the PD-1 inhibitors sub-segment held the largest share in 2024. This is mainly due to their broad effectiveness across various cancers, manageable side effects, and proven clinical success, especially compared to earlier checkpoint inhibitors like CTLA-4 inhibitors. PD-1 inhibitors such as pembrolizumab and nivolumab have shown substantial efficacy in treating melanoma, lung cancer, head and neck cancers, and others, which has helped them become widely adopted by restoring the body's natural anti-tumor immunity.
The bispecific antibodies segment is expected to grow at the highest CAGR over the projection period. This is because of the unique ability of bispecific antibodies, especially T-cell engagers (BiTEs), to redirect T-cells to attack cancer cells, providing a targeted and potent immunotherapy approach. BiTEs bind simultaneously to a tumor-associated antigen on cancer cells and the CD3 receptor on T-cells, effectively bringing the T-cell close to the cancer cell for targeted destruction. This approach can overcome the suppressive tumor microenvironment by recruiting and activating T-cells, even in cases where other immunotherapies might fail, showing promising results.
Application Insights
How Does the Non-Small Cell Lung Cancer (NSCLC) Segment Dominate the Immuno-Oncology Antibodies Market in 2024?
The non-small cell lung cancer (NSCLC) segment led the market with a significant share in 2024, mainly because of advancements in immunotherapy and the high incidence and mortality rates associated with NSCLC. As the most common lung cancer type and a leading cause of cancer-related death worldwide, this disease's burden drives considerable research and development efforts, including exploring immuno-oncology strategies. Immunotherapies like checkpoint inhibitors, nivolumab, pembrolizumab, atezolizumab, and durvalumab targeting PD-1/PD-L1 and CTLA-4 have achieved remarkable success in treating advanced NSCLC, improving patient survival outcomes.
The triple-negative breast cancer (TNBC) segment is likely to grow at a rapid pace in the upcoming period due to its aggressive nature, lack of targeted therapies for TNBC, and promising results from immunotherapy, especially immune checkpoint inhibitors. Unlike other breast cancer types, TNBC does not express ER, PR, or HER2, making traditional targeted therapies ineffective. Immune checkpoint inhibitors like pembrolizumab (Keytruda) have demonstrated significant improvements in pathological complete response (pCR) rates when combined with neoadjuvant chemotherapy in early-stage TNBC.
Mechanism of Action Insights
Why Did the Immune Checkpoint Blockade Segment Dominate the Immuno-Oncology Antibodies Market in 2024?
The immune checkpoint blockade segment dominated the market with a major share in 2024 due to its remarkable success in harnessing the immune system to fight cancer, resulting in significant clinical benefits and applications across many indications. These inhibitors have shown notable efficacy in melanoma, lung cancer, Hodgkin's lymphoma, and other cancers, bringing paradigm shifts in treatment strategies. They have demonstrated durable responses and often outperform traditional therapies, boosting long-term disease control and, in some cases, potential cures, an important advantage over other treatment options.
The immune cell redirection segment is expected to experience the fastest growth in the market, mainly because of its ability to harness the body's immune system to target and destroy cancer cells. It redirects immune cells like T cells and NK cells to recognize and eliminate tumors, offering a more targeted and potentially less toxic treatment compared to traditional therapies. This approach can be combined with other immunotherapies, such as checkpoint inhibitors, or with conventional treatments like chemotherapy or radiation, to boost their effectiveness and expand their potential applications, supporting ongoing research and development.
Route of Administration Insights
What Made Intravenous (IV) the Dominant Segment in the Immuno-Oncology Antibodies Market in 2024?
The intravenous (IV) segment dominated the market in 2024. This is mainly due to the proven efficacy, broad applicability, and capacity of IV route of administration to deliver high doses directly into the bloodstream for rapid distribution throughout the body. Although subcutaneous administration is gaining popularity, the established success of IVs and the need for precise dosing in immuno-oncology still make it the preferred route for many therapies. Moreover, IV allows for accurate control of the dose, ensuring the intended amount of the therapeutic antibody reaches the bloodstream, a crucial factor in immuno-oncology, where precise targeting and adequate drug levels are vital for optimal results.
The subcutaneous (SC) segment is expected to expand a the highest CAGR during the forecast period due to its higher patient convenience and compliance. Switching to SC administration can free up hospital resources, lessen the demand for specialized infusion centers, and shorten hospital stays. SC injections can be given much faster than IV infusions, especially for monoclonal antibodies (mAbs), which often take hours to infuse. The development of prefilled syringes, autoinjectors, and other devices has made SC administration more user-friendly and accessible, particularly for self-administration, enhancing its benefits.
End User Insights
How Does the Hospitals Segment Lead the Immuno-Oncology Antibodies Market in 2024?
The hospitals remained the dominant segment in 2024 because of their central role in cancer care, covering diagnosis, treatment, and clinical trial enrollment. They are equipped with advanced infrastructure and skilled staff to manage the complex nature of immune-oncology therapies, which often involve intravenous infusion and intensive monitoring. Hospitals also tend to lead in adopting new therapies due to their research facilities and advanced infrastructure. The presence of oncologists, nurses, pharmacists, and other specialists ensures proper treatment planning and management, which is critical for immune-oncology therapies. As a result, hospitals continue to play a vital role in conducting clinical trials for these therapies, reinforcing their market position.
The cancer specialty clinics segment is expected to register the fastest CAGR in the coming years. The growth of the segment can be attributed to the increasing cancer prevalence, advances in personalized medicine, and the effectiveness of immune-oncology therapies. Specialized clinics focused on specific cancer types, like hematological cancers, are well-equipped to deliver complex immune-oncology treatments and provide expert care. The rising demand for personalized medicine and patient-specific care also contributes to segmental growth.
Distribution Channel Insights
Why Did the Hospital Pharmacies Segment Dominate the Immuno-Oncology Antibodies Market in 2024?
The hospital pharmacies segment dominated the market while holding the largest share in 2024. This is mainly due to the increased hospital admissions rates for cancer treatment, the complexity of administering cancer therapies, and patients' preference for receiving treatment in hospital settings. Since many immuno-oncology antibodies need specialized administration, such as intravenous infusions, hospitals are the preferred locations for delivery. Additionally, cancer patients often require a multidisciplinary team and advanced diagnostics, which are readily available in hospitals. Government initiatives supporting cancer centers and research also support hospital pharmacies' growth in this market.
The specialty pharmacies segment is expected to grow at a significant rate because of their ability to handle the complex demands of these therapies, including specialized handling, distribution, and patient support services. The growth of the segment is also driven by the rising number of cancer cases, advances in immune-oncology treatments, and the unique requirements of these therapies. Specialty pharmacies offer comprehensive patient support, such as adherence programs, financial assistance guidance, and clinical expertise, which are essential for managing side effects and therapy complexities. Pharmaceutical companies are increasingly partnering with specialty pharmacies to optimize drug distribution and improve patient outcomes, ensuring patients have access to these advanced therapies.
Regional Insights
What Made North America the Dominant Region in the Immuno-Oncology Antibodies Market in 2024?
North America registered dominance in the immuno-oncology antibodies market by capturing the largest share in 2024. This is mainly due to its well-established healthcare infrastructure, high cancer prevalence, and increased research and development activities. North America is a hub for cutting-edge research in immuno-oncology, with numerous pharmaceutical companies and research institutions actively developing and testing new therapies. Regulatory bodies like the FDA expedite the approval process for new immuno-oncology drugs, enabling faster market access. The region's dominance is also supported by the presence of many leading players such as Pfizer, Amgen, Bristol Myers Squibb, and others. Additionally, greater patient awareness and quicker adoption of new therapies contribute to the region's leadership.
In May 2025, the FDA granted accelerated approval to AbbVie's Emrelis (telisotuzumab vedotin-tllv) for treating adults with previously treated, locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) that exhibits high c-Met protein overexpression. Emrelis is the only approved therapy with results from the Phase II LUMINOSITY trial, which showed a 35% overall response rate and a median duration of response of 7.2 months. (Source: https://www.pharmexec.com)
U.S. Immuno-Oncology Antibodies Market Trends
The U.S. is a major contributor to the market. The country's position in the market is supported by substantial government funding for oncology research, a strong presence of major pharmaceutical companies like Merck and Bristol-Myers Squibb, and a favorable regulatory environment facilitated by the FDA. The high rates of cancer in the U.S. and the rising demand for effective therapies further propel market growth, with numerous clinical trials and FDA approvals fostering advancements in the field.
What Makes Asia Pacific the Fastest-Growing Market for Immuno-Oncology Antibodies?
Asia Pacific is expected to experience the fastest growth during the forecast period. This growth is mainly driven by rising cancer rates, increasing awareness of advanced therapies, and supportive government initiatives in countries like China, India, and Japan. Technological advancements, particularly in personalized medicine and immune checkpoint inhibitors, are also fueling regional market growth. Governments around the region are implementing policies to promote research, development, and commercialization of immuno-oncology products, including tax incentives and supportive measures. The region offers competitive labor costs, well-developed infrastructure, and access to a large patient pool, making it an attractive location for clinical trials and drug development, contributing to market growth.
India Immuno-Oncology Antibodies Market Trends
India is emerging as a significant player in the market, mainly due to increasing cancer rates, growing adoption of immunotherapy, and supportive government policies. The rise in cases of various cancers, including lung and breast cancers, which are particularly responsive to immunotherapy, drives this growth. Government funding and initiatives, along with advances in biotechnology and research, foster a strong R&D environment and facilitate collaborations between local and global pharmaceutical companies, improving access to life-saving treatments both domestically and internationally.
In April 2024, India launched its first domestically developed anti-cancer CAR-T cell therapy at IIT Bombay, with President Smt. Droupadi Murmu in attendance. This affordable therapy was created through collaboration among IIT Bombay, Tata Memorial Centre, and industry partner ImmunoACT. The launch was celebrated as a major milestone in India's healthcare innovation, highlighting successful academia-industry partnerships.
Why is Europe Considered a Notable Region in the Immuno-Oncology Antibodies Market?
Europe is expected to experience notable growth over the projection period, fueled by robust research and development infrastructure, strong regulatory frameworks, and rising awareness of cancer immunotherapy. The increase in cancer incidence, higher investment in research, and adoption of personalized medicine approaches also contribute. Governments and private investors are significantly boosting funding for cancer research, including immuno-oncology, especially in countries like Germany, France, and the UK. A clear and supportive regulatory landscape within the EU further encourages innovation and speeds up approval processes for new immune-oncology treatments.
What Opportunities Exist in Latin America?
Latin America is expected to emerge as a major player in the market because of rising cancer rates, increased awareness of immunotherapy, and expanding access to advanced treatments. Governments in Brazil, Argentina, and Colombia are investing heavily in healthcare infrastructure and research. Government initiatives to improve access to innovative cancer therapies further support regional market growth. Simplified regulatory procedures and faster approval times for immuno-oncology drugs are helping new therapies enter the market. Additionally, efforts to implement precision oncology, tailoring treatments based on individual patient characteristics, are gaining momentum, further fueling the growth of the market.
What Factors Contribute to the Immuno-Oncology Antibodies Market in the Middle East & Africa?
The market in the Middle East & Africa is expected to grow due to the high prevalence of chronic diseases like cancer and autoimmune disorders, increased investments in healthcare facilities, and research. Government initiatives, including national cancer screening programs, higher healthcare spending, and efforts to improve access to advanced treatments, are fostering market growth. Many countries in this region, like the UAE, Saudi Arabia, and Egypt, are improving their healthcare systems through national programs and improved access to diagnostic and treatment facilities, especially within the Gulf Cooperation Council region.
Immuno-Oncology Antibodies Market Companies

- Bristol Myers Squibb
- Merck & Co., Inc.
- Roche Holding AG
- AstraZeneca
- Pfizer Inc.
- Regeneron Pharmaceuticals
- Sanofi
- Novartis AG
- Amgen Inc.
- Gilead Sciences (Incl. Kite Pharma)
- Eli Lilly and Company
- BeiGene Ltd
- Innovent Biologics
- MacroGenics
- Zymeworks Inc.
- Genmab A/S
- Seagen Inc.
- I-Mab Biopharma
- Hengrui Medicine
- Alphamab Oncology
Recent Developments
- In November 2025, Dr. Reddy's Laboratories launched Toripalimab, the first immuno-oncology drug for nasopharyngeal carcinoma in India. This PD-1 inhibitor provides a new treatment option for this rare head and neck cancer, which previously relied on chemotherapy. It reduces the risk of progression or death by 48%. Branded as Zytorvi in India, Toripalimab is the only immuno-oncology drug approved for recurrent or metastatic nasopharyngeal carcinoma by major global regulatory authorities.
(Source: https://timesofindia.indiatimes.com) - In June 2025, BioNTech SE and Bristol Myers Squibb announced a partnership for the global co-development and co-commercialization of BioNTech's bispecific antibody BNT327, targeting PD-L1 and VEGF-A across various solid tumors. This includes Phase 3 trials for extensive-stage small cell lung cancer (ES-SCLC) and non-small cell lung cancer (NSCLC), with a Phase 3 trial for triple-negative breast cancer (TNBC) set to begin by the end of 2025. The collaboration aims to broaden treatment options and explore combinations with other therapies. (Source: https://news.bms.com)
- In April 2025, Biotherapeutics, Inc. and LigaChem Biosciences formed a strategic partnership to develop novel payloads for antibody-drug conjugates (ADCs) and small-molecule immuno-oncology therapies. They plan to identify and develop 2–4 new drug candidates over three years, with LigaChemBio providing initial funding and holding exclusive licensing options. This partnership builds on previous research collaborations to advance ADC payloads and small-molecule treatments in oncology.
(Source: https://www.pharmoutsourcing.com) - In March 2025, the U.S. FDA approved Imfinzi (durvalumab) in combination with chemotherapy for adults with muscle-invasive bladder cancer, allowing its use before and after surgery. This approval was based on the NIAGARA Phase III trial, which involved 1,063 patients and assessed the effectiveness of neoadjuvant durvalumab with chemotherapy followed by adjuvant durvalumab after surgery. (Source: https://www.fda.gov)
Segments Covered in the Report
By Drug Class
- Checkpoint Inhibitors
- PD-1 Inhibitors
- PD-L1 Inhibitors
- CTLA-4 Inhibitors
- LAG-3 Inhibitors
- TIGIT Inhibitors
- Others
- Bispecific Antibodies
- T-cell Engagers (BiTEs)
- Dual Immune Checkpoint Blockers
- Dual Tumor Targeting
- Others
- Immune Agonist Antibodies
- OX40 Agonists
- CD137 (4-1BB) Agonists
- Others
- Antibody-Drug Conjugates (ADCs)
- Targeted Payload Carriers
- Immune-Stimulating ADCs
- Others
- Tumor-targeted mAbs with ADCC/CDC
- Radiolabeled Antibodies
- Fusion Protein-Antibody Hybrids
By Application (Cancer Type)
- Non-Small Cell Lung Cancer (NSCLC)
- Melanoma
- Renal Cell Carcinoma
- Head and Neck Cancer
- Triple-Negative Breast Cancer (TNBC)
- Colorectal Cancer (CRC)
- Hepatocellular Carcinoma (HCC)
- Hematologic Malignancies (Lymphoma, Leukemia, Myeloma)
- Bladder Cancer
- Gastric and Esophageal Cancer
- Cervical and Endometrial Cancer
- Others (Pancreatic, Brain Tumors, Rare Cancers)
By Mechanism of Action
- Immune Checkpoint Blockade
- Immune Cell Redirection
- Tumor Antigen Targeting with Immune Effector Recruitment
- Cytokine Pathway Modulation
- Immune Stimulation via Receptor Agonism
- Combination of Immune Activation and Cytotoxicity
By Route of Administration
- Intravenous (IV)
- Subcutaneous (SC)
- Others (e.g., Intratumoral, Intraperitoneal)
- End User
- Hospitals
- Cancer Specialty Clinics
- Academic and Research Institutions
- Others (Outpatient Centers, Homecare)
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Specialty Pharmacies
- Others (Clinical Trial Supply, Direct-to-Patient)
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting