Clinical Trial Biorepository and Archiving Solutions Market Size, Report 2032
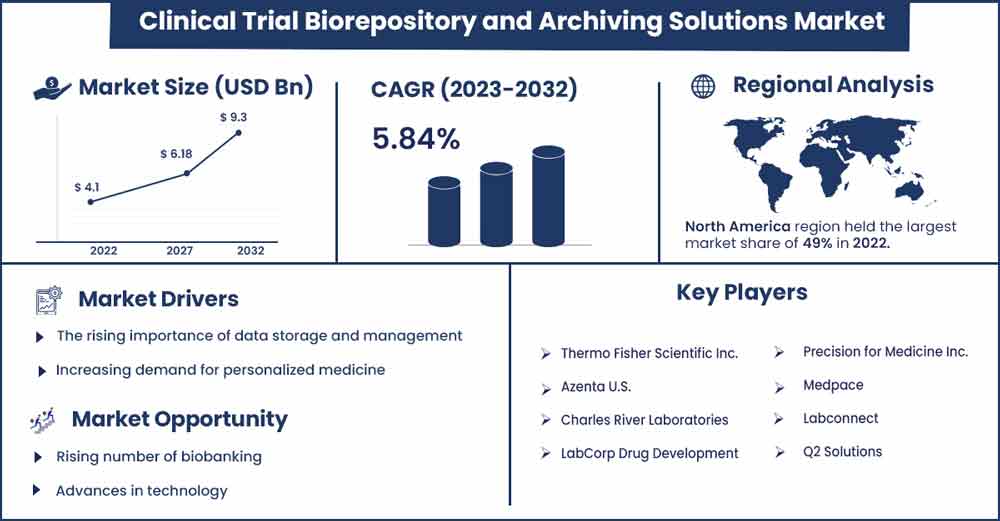
The global clinical trial biorepository and archiving solutions market size was exhibited at USD 4.1 billion in 2022 and is projected to attain around USD 9.3 billion by 2032, growing at a CAGR of 5.84 % during the forecast period 2023 to 2032.
Improving the infrastructure of the biotechnology industry across the globe is observed to drive the growth of the clinical trial biorepository and archiving solutions market.
Market overview:
A clinical trial biorepository is a collection of biological samples, such as blood, tissue, urine, or saliva, collected from patients during a clinical trial. Biorepositories can help to improve the quality and accuracy of data collected during the trial, enable further analysis of biomarkers, and help researchers identify new targets for drug development. Archiving solutions refer to the storage and management of clinical trial data and samples, including those stored in biorepositories. These solutions ensure that data and samples are stored in a secure and controlled environment and that researchers can access them quickly and efficiently. These solutions often include features such as access controls, data backups, and versioning to ensure data integrity and prevent loss or corruption of data.
The clinical trial biorepository and archiving solutions market is driven by factors such as increasing funding for clinical trials, rising demand for personalized medicine, and the growing importance of data management and storage in clinical research. Additionally, technological advancements, such as automated sample processing systems and cloud-based storage solutions, have contributed to the market's growth.
Report Highlights:
- By product, the clinical segment dominated the market in 2022; the segment will continue to grow with the rising demand for healthcare services with an increasing willingness to invest in healthcare products. On the other hand, the preclinical segment will witness a noticeable increase owing to the rising demand for the research and development of novel drugs, especially for chronic diseases in the global market.
- By phase, phase III holds the dominating share of the market. The segment's growth is attributed to the fact that phase III clinical trials require a reliable facility to store and manage specimens and data, whereas biorepository and archiving solutions offer the same integrity. On the other hand, the phase II segment is expected to register significant growth during the forecast period owing to the rising investments in clinical trials as well as research and development activities.
- By services, the biorepository services segment held the dominating share in 2022; the segment will maintain its dominance through the forecast period. The segment's growth is significantly driven by the increased demand for storage facilities for biosamples across the globe. On the other hand, with rising clinical trials, the archiving solutions segment is predicted to witness noticeable growth during the analyzed period.
Clinical Trial Biorepository and Archiving Solutions Market Report Scope:
| Report Coverage | Details |
| Market Revenue in 2023 | USD 4.45 Billion |
| Projected Forecast Revenue by 2032 | USD 9.3 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 5.84% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Regional Snapshot:
North America held the dominating share of the global clinical trial biorepository, and archiving solutions market, and the region is expected to maintain its dominance during the analyzed period due to the rapid adoption of modern technology for clinical trials and even research and development activities.
Moreover, the rising demand for personalized medicine in North America is observed to supplement the market’s growth. Biorepository facilities play a crucial role in precision medicine by providing a source of high-quality biological samples for research and development.
Some of the key players operating in the North American clinical trial biorepository and archiving solutions market include Thermo Fisher Scientific Inc., BioLife Solutions, Inc., Hamilton Company, VWR International LLC, Brooks Automation, Inc., and Chart Industries, Inc. These companies are investing heavily in research and development to develop innovative biorepository and archiving solutions and gain a competitive edge in the market.
Asia Pacific is expected to witness the fastest growth in the clinical trial biorepository and archiving solutions market due to a rising focus on biosimilar product development. China, India, and Japan are expected to continue contributing to the market’s growth during the forecast period. India is a significant market for clinical trial biorepository and archiving solutions, with the country's large and diverse population providing ample opportunities for clinical research.
On the other hand, Japan is a mature market for clinical trial biorepository and archiving solutions, with the country home to several leading pharmaceutical and biotechnology companies. The Japanese government's focus on precision medicine and the increasing adoption of digital technologies in clinical trials are driving the growth of the market in the country.
Europe is expected to remain the most attractive region for the clinical trial biorepository and archiving solutions market. The United Kingdom is a significant market for clinical trial biorepository and archiving solutions, with the country's National Health Service (NHS) providing a large and diverse population for clinical research. Additionally, the UK government's investments in research and development in the healthcare sector, including initiatives such as the Life Sciences Sector Deal and the Industrial Strategy Challenge Fund, are driving market growth in the country. Moreover, the French government has also made significant investments in research and development in the healthcare sector, which is driving the growth of the market in the country.
Market Dynamics:
Driver:
Increasing demand for personalized medicine
Personalized medicine relies on understanding the genetic makeup of individual patients to develop targeted therapies. This requires high-quality patient biological samples, which can be stored and managed in biorepositories. The demand for personalized medicine is driving an increase in clinical trials and, therefore, the need for biorepositories to store and manage these samples. Overall, the rising demand for personalized medicine is driving the growth of the clinical trial biorepositories and archiving solutions market by increasing the need for high-quality biological samples, improving data collection and management, enhancing sample tracking and analysis, and facilitating greater collaboration and sharing of data. As personalized medicine continues to grow in importance, the demand for biorepositories and archiving solutions is expected to increase as well.
Restraint:
Lack of skilled workforce
Biorepository and archiving solutions require a skilled workforce to operate and manage them. Without sufficiently skilled personnel, the efficiency of biorepository and archiving processes can be compromised, leading to delays and errors. A skilled workforce is necessary to ensure that biorepository and archiving solutions are operating at the highest level of quality. A skilled workforce is necessary to develop new and innovative biorepository and archiving solutions that can meet the changing needs of the clinical trial industry. The shortage of skilled personnel can limit the ability of companies to innovate in this area. Thus, the shortage or lack of skilled workforce is considered a major restraining factor for the market’s growth.
Opportunity:
Advances in technology
Advances in technology are observed to offer a plethora of opportunities to the market players in the clinical trial biorepository and archiving solutions market by improving efficiency, accuracy, and accessibility. As technology continues to evolve, the market players can expect to see further growth and innovation in the market, resulting in better patient outcomes and more efficient clinical research. Technological advancements such as cloud-based solutions are becoming increasingly popular in the biorepository and archiving solutions market. These solutions offer secure, flexible, and scalable storage for large amounts of data that can be accessed anywhere. Moreover, automation is increasingly being used in biorepositories to streamline the process of storing and managing biological samples. This has led to increased efficiency and reduced the need for manual labor, resulting in cost savings.
Challenge:
Regulatory compliance
Biorepository and archiving solutions must comply with various regulations and guidelines, including Good Clinical Practice (GCP), Good Manufacturing Practice (GMP), and Good Laboratory Practice (GLP). Maintaining compliance can be challenging and time-consuming for market players, which creates an obstacle to the market’s growth.
Recent Developments:
- In February 2021, Cincinnati Children’s Hospital Medical Center announced its collaboration with Down Syndrome Achieves (DSA) in order to launch a research biobank. The biobank will serve as a central repository for collected biospecimen donated by people with down syndrome and their families.
- In February 2023, the Philadelphia-based Fox Chase Cancer Center Biosample Repository Facility received accreditation from the College of America Pathologists. The award is granted for the facility’s tremendous efforts in processing, storing, disseminating, and analyzing biosamples.
- In January 2022, based in Hyderabad, India, L V Prasad Eye Institute announced the opening of the Ophthalmic Research Biorepository, a first-of-its-kind facility in the country. The biorepository facility is equipped with advanced infrastructure required for the cryopreservation of ophthalmic tissues at very low temperatures.
Major Key Players:
- Thermo Fisher Scientific Inc.
- Azenta U.S.
- Charles River Laboratories
- LabCorp Drug Development
- Precision for Medicine Inc.
- Medpace
- Labconnect
- Q2 Solutions
Market Segmentation:
By Product
- Preclinical Products
- Clinical Products
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Services
- Biorepository Services
- Archiving Services
Buy this Research Report@ https://www.precedenceresearch.com/checkout/2749
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 9197 992 333