Pulmonary Alveolar Proteinosis (PAP) Drugs Market Revenue to Attain USD 1,381.26 Mn by 2033
Pulmonary Alveolar Proteinosis (PAP) Drugs Market Revenue and Trends 2025 to 2033
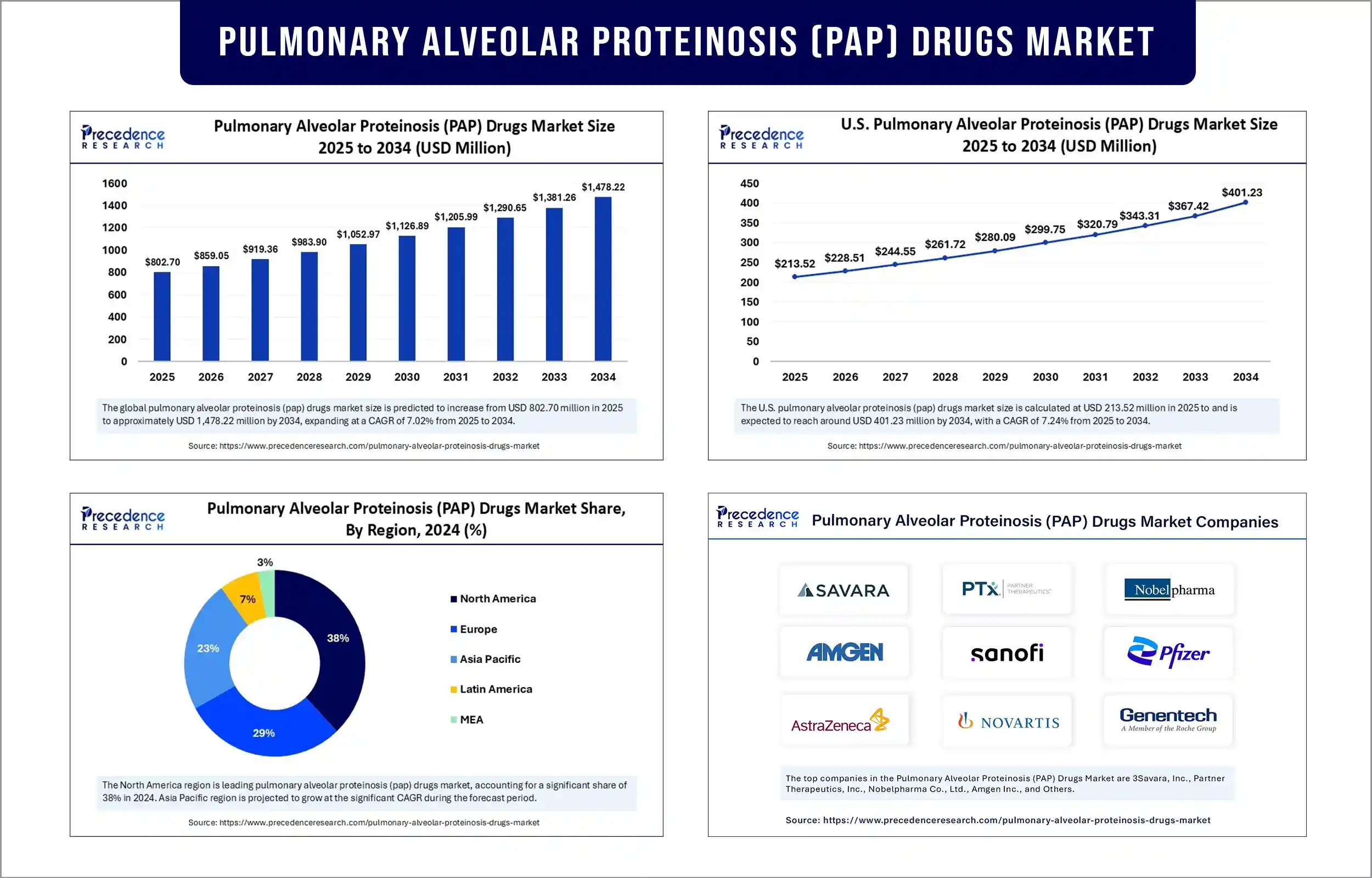
The global pulmonary alveolar proteinosis (PAP) drugs market revenue was valued at USD 802.70 million in 2025 and is expected to attain around USD 1,381.26 million by 2033, growing at a CAGR of 7.02% during forecast period. This market is expanding due to the increasing prevalence of PAP, advancements in targeted therapies like inhaled GM-CSF, and growing patient awareness.
What are the Key Factors Boosting the Growth of the Pulmonary Alveolar Proteinosis (PAP) Drugs Market?
The pulmonary alveolar proteinosis (PAP) drug market is experiencing robust growth, driven by several key factors. Firstly, the rising incidence of autoimmune PAP, coupled with improved diagnostic tools that facilitate quicker and more precise diagnoses, has expanded the patient base seeking treatment. Concurrently, advancements in therapeutic options, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) replacement therapy and the enhanced patient responses seen with targeted biologics like rituximab, are influencing the market. Furthermore, heightened awareness of this rare lung disease, combined with the demand for specialized therapies from both healthcare providers and patients, is driving market growth. Favorable regulatory environment, increased investments in research and development, and the rising demand for personalized therapies are likely to contribute to market expansion in the near future.
Segment Insights
- By therapeutic class/drug modality, the inhaled recombinant GM-CSF segment sustained dominance in the market while holding the largest share in 2024, as it provides effective, non-invasive treatment. It offers a safer and patient friendly alternative to the traditional standard-of-care, whole-lung lavage (WLL), improving lung function and diminishing reliance on whole-lung lavage.
- By formulation & delivery, the nebulized aqueous solutions segment dominated the market in 2024. This is mainly due to their better efficacy and improved patient compliance. These solutions can deliver GM-CSF directly into the lungs via aerosol, allowing higher local drug concentration at the diseased site with fewer systemic adverse effects, showing superior efficacy compared to subcutaneous routes.
- By indication/PAP type, the autoimmune pulmonary alveolar proteinosis (aPAP) segment led the market in 2024. Due to the rarity of this condition, there is a heightened need for targeted treatments. Increasing diagnosis rates further support segmental growth.
- By treatment setting, the hospital/specialty pulmonary centers segment dominated the pulmonary alveolar proteinosis (PAP) drugs market in 2024 due to their key role in managing this rare and complex condition. These settings also boast advanced diagnostic tools, such as HRCT and bronchoalveolar lavage, and specialized treatments such as whole-lung lavage and GM-CSF therapy, increasing the patient pool.
- By patient segment/use case, the recurrent/relapsing disease is the dominant segment in the market, as relapsing cases require ongoing therapeutic intervention, driving consistent demand for PAP drugs. Additionally, recurrent PAP patients tend to require more frequent follow ups, imaging, and advanced monitoring, which increases healthcare utilization and spending on drug therapies over time.
Regional Insights
North America registered dominance in the pulmonary alveolar proteinosis (PAP) drugs market, capturing nearly 35-40% share in 2024. This is mainly due to its robust healthcare infrastructure, heightened awareness of rare diseases, and significant research funding. Specialized hospitals with pulmonary centers and experienced physicians enable advanced PAP treatments, including whole lung lavage and inhaled GM-CSF therapies. Ongoing research studies on rare diseases by specialty hospitals, universities, and pharmaceutical companies, which are focused on refining existing treatments and developing innovative therapeutic options, further ensure the long-term growth of the market in the region.
Asia Pacific is expected to experience rapid growth in the market, driven by heightened awareness, improved diagnostic efficiency, and rising healthcare infrastructure development. Japan, China, and India, for example, are witnessing high demand for inhaled GM-CSF therapies. The rising development of specialized pulmonary centers across the region, especially in emerging countries, and regulatory support are likely to drive market growth. In addition, the rising prevalence of rare diseases and the development of innovative treatments further contribute to regional market growth.
Pulmonary Alveolar Proteinosis (PAP) Drugs Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 802.70 Million |
| Market Revenue by 2033 | USD 1,381.26 Million |
| CAGR from 2025 to 2033 | 7.02% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Development
- In 2024, Japan's pharmaceutical authorities approved sargramostim, a recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), for inhalation therapy in patients with autoimmune pulmonary alveolar proteinosis (aPAP).
(Source: https://www.sciencedirect.com)
Pulmonary Alveolar Proteinosis (PAP) Drugs Market Key Players
- Savara Inc
- Partner Therapeutics, Inc.
- Nobelpharma Co., Ltd.
- Amgen Inc.
- Sanofi (historical owner/partner for GM-CSF assets)
- Pfizer Inc.
- AstraZeneca plc
- Novartis AG
- Roche / Genentech
- Johnson & Johnson (Janssen)
- Takeda Pharmaceutical Company Limited
- Mallinckrodt Pharmaceuticals
- Baxter International Inc.
- Biogen Inc.
- Regeneron Pharmaceuticals, Inc.
- GlaxoSmithKline plc (GSK)
- Teva Pharmaceutical Industries Ltd.
- Viatris / Mylan
- Specialty CDMOs & biologics manufacturers
- Rare-disease / pulmonary biotech specialists
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6635
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344