Veterinary CRO and CDMO Market Size To Rise USD 13.60 Bn By 2032
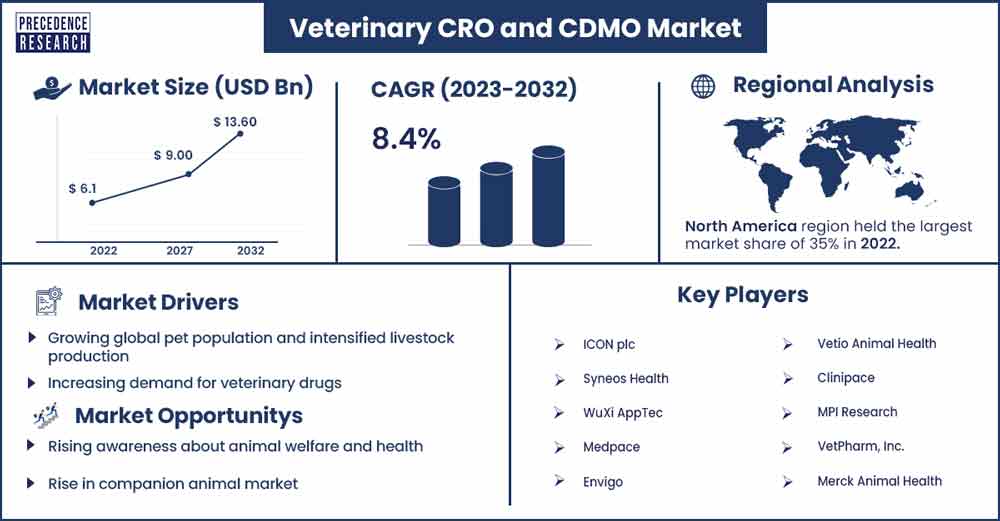
The global Veterinary CRO and CDMO market size surpassed USD 6.1 billion in 2022 and is projected to rise to USD 13.60 billion by 2032, anticipated to grow at a CAGR of 8.4 percent during the projection period from 2023 to 2032.
Market Overview
The service offered to the animal health business for the study, creation, and production of veterinary medications, vaccines, and associated goods is referred to as the veterinary contract research organization (CRO) and contract development and manufacturing organization (CDMO) market. Regulatory support, clinical trials, and preclinical testing are among the services veterinary CROs provide to support the development of veterinary medications. These services contribute to the efficacy and safety of items used in animal health.
Veterinary CDMOs offer services for producing and developing drugs and vaccines used in the animal health sector. They support both regulatory compliance and production scaling. Some CROs and CDMOs specialize in animal health services, making the market competitive. This industry frequently collaborates with biotech, pharmaceutical, and other animal health-related enterprises.
Due to rising pet ownership and growing pet healthcare knowledge, the need for CRO and CDMO services has been fuelled by increased demand for veterinary services and pharmaceuticals. The market for CROs and CDMOs specializing in veterinary medicines has grown due to the development of novel medications and treatments for companion animals, such as dogs, cats, and horses. Pharmaceuticals and biotech businesses are outsourcing more research and development work to cut costs, which acts as a growth factor for the market. Pharmaceutical businesses and startups are turning to CROs and CDMOs for specialized knowledge due to strict regulations and the need to comply with safety and effectiveness criteria.
- In September 2022, Mitsui & Co., Ltd. stated to purchase 29.44% of the shares of Ouro Fino Saude Animal Participacoes S.A., a Brazilian animal health company. Ouro Fino is present throughout Central and South America and its home market of Brazil in international trade. It is the fourth-biggest animal health company in Brazil that creates, produces, and markets veterinary medications for companion and livestock animals.
Regional Snapshot
North America holds the largest share of the veterinary CRO and CDMO market. Due to the rising need for medications, vaccinations, and other animal healthcare supplies, the North American veterinary CRO and CDMO business is expanding robustly. The market includes various services, such as clinical trial development, drug development, formulation development, and veterinary product manufacturing.
Veterinary medications and healthcare goods are in high demand due to the growing pet population and awareness of pet health. Companies sought specialized CRO and CDMO services to comply with the strict regulatory standards for veterinary medication development and production.
The biotechnology, pharmaceutical, and animal health sectors are the main customers of the US veterinary CRO and CDMO market. These companies provide services for creating, examining, and producing veterinary medications and goods. The veterinary CRO and CDMO market has grown due to increased demand for cutting-edge medications and healthcare supplies. The veterinary industry is investing more in research and development due to a focus on companion animal health, livestock productivity, and food safety. Preclinical and clinical trials, toxicological investigations, regulatory advice, and data administration are among the services given by CROs to the veterinary industry.
Within the region’s pharmaceutical and biotechnology sector, Canadian veterinary CRO and CDMO market is a major marketplace. It includes companies that offer services for the production, manufacturing, and research & development of veterinary medications and related goods. The demands of businesses creating medications, vaccinations, and other animal healthcare goods are met by this market.
The need for veterinary medications and healthcare supplies is growing as more people in Canada keep pets, such as dogs, cats, and exotic animals. The demand for new and improved medications and treatments has motivated research and development efforts in the veterinary industry. Tight regulatory requirements drive the need for R&D and manufacturing skills, causing the expansion of CRO and CDMO services.
North America has several players in the veterinary CRO and CDMO market, including Charles River Laboratories, Inc., Clinvet, KLIFOVET AG, Löhlein & Wolf Vet Research, Oncovet Clinical Research, ONDAX Scientific, Triveritas, Veterinary Research Management (VRM) Ltd., VetPharm, Inc.
- In September 2023, a multi-program collaboration deal was revealed between CRL and Related Science, a data science-driven drug development company. As a result of this collaboration, Logica, the joint product of Charles River and Valo Health, will be used on a number of Related Science hitherto unexplored objectives.
Veterinary CRO and CDMO Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 6.58 Billion |
| Projected Forecast Revenue by 2032 | USD 13.60 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 8.4% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Research activities
An increasing pet population and growing awareness of animal health are driving up demand for pharmaceuticals, particularly medications and immunizations for animals. This fuelled the veterinary industry's need for research and development services. More and more biotech and pharmaceutical businesses are outsourcing their manufacturing and research to veterinary-focused CROs and CDMOs. Like its counterpart in human health, the veterinary pharmaceutical business is subject to stringent regulatory regulations. To make sure that products fulfil these requirements and are safe for use on animals, CROs and CDMOs are essential.
Increasing demand for veterinary drugs
Increased funding for research and development results from the demand for novel and efficient veterinary medications. Veterinary CROs conduct preclinical research and clinical trials to support this demand. To comply with regulatory standards and guarantee the safety and effectiveness of veterinary medications, veterinary CROs and CDMOs must possess specific knowledge in veterinary drug development. The global market for CRO and CDMO services in the veterinary industry is growing as the demand for veterinary medications rises.
Restraints
High development costs
Adherence to regulatory rules and numerous safety and efficacy standards is crucial when developing veterinary medications and medical devices. The process of demonstrating safety and efficacy through thorough preclinical and clinical investigations is a complex and costly regulatory approach for veterinary products. Developing veterinary medications and medical equipment requires a significant investment in research and development (R&D). Rigorous testing and validation procedures frequently ensure veterinarian product safety and effectiveness. The expenditures are increased by ethical issues surrounding the use of animals in research and testing.
Limited market landscape
The veterinary pharmaceutical industry is comparatively more minor than the pharmaceutical sector for humans. This is because of a lesser selection of drugs and treatments required to maintain the health of animals. Companies in the veterinary business could have fewer funds for research and development than those in the human pharmaceutical industry. The resources available to CROs and CDMOs for service outsourcing may be limited. Products for companion animals, such as cats and dogs, dominate the veterinary pharmaceutical market.
Opportunities
Rising awareness about animal welfare and health
Veterinary medications are becoming more and more necessary as more people acquire pets and care for them worldwide. This covers the more common pets like dogs and cats and the growing trend of people being interested in rare and niche species. The value of animal health and welfare is becoming more recognized by livestock producers and pet owners. This has increased the need for cutting-edge veterinary medications to treat various animal health problems. Quality assurance and regulatory compliance are becoming increasingly important in the veterinary pharmaceutical sector.
Rise in companion animal market
The market for companion animals is expanding significantly in tandem with rising pet ownership and pet care expenditures. There brings an increasing need for premium veterinary medicines, biologics, and healthcare products due to the significance of pets in people's lives. Preclinical testing, clinical trials, safety and efficacy evaluations, and regulatory support are among the services veterinary CROs provide to the companion animal industry for medication development. Companion animal sales include many animals, such as horses, dogs, cats, and other species. The unique requirements of these animals must frequently be catered for in products.
Recent Developments
- In June 2023, Union Minister, India announced the launch of Nandi Portal for Indian veterinary industry that focuses on the process of getting easy approval for veterinary drugs.
Major key Players
- Covance Inc. (a LabCorp Company)
- Charles River Laboratories International, Inc.
- PAREXEL International Corporation
- ICON plc
- Pharmaceutical Product Development, LLC (PPD)
- Syneos Health
- WuXi AppTec
- Medpace
- Envigo
- Vetio Animal Health
- Clinipace
- MPI Research
- Veterinary Research Management (VRM)
- VetPharm, Inc.
- Merck Animal Health
Market Segmentation
By Animal Type
- Companion Animals
- Livestock Animals
By Service Type
- Discovery
- Development
- Manufacturing
- Packaging & Labeling
- Market Approval & Post-marketing
By Application
- Medicines
- Medical Devices
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3353
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 9197 992 333