Psoriasis Biosimilars Market Size?
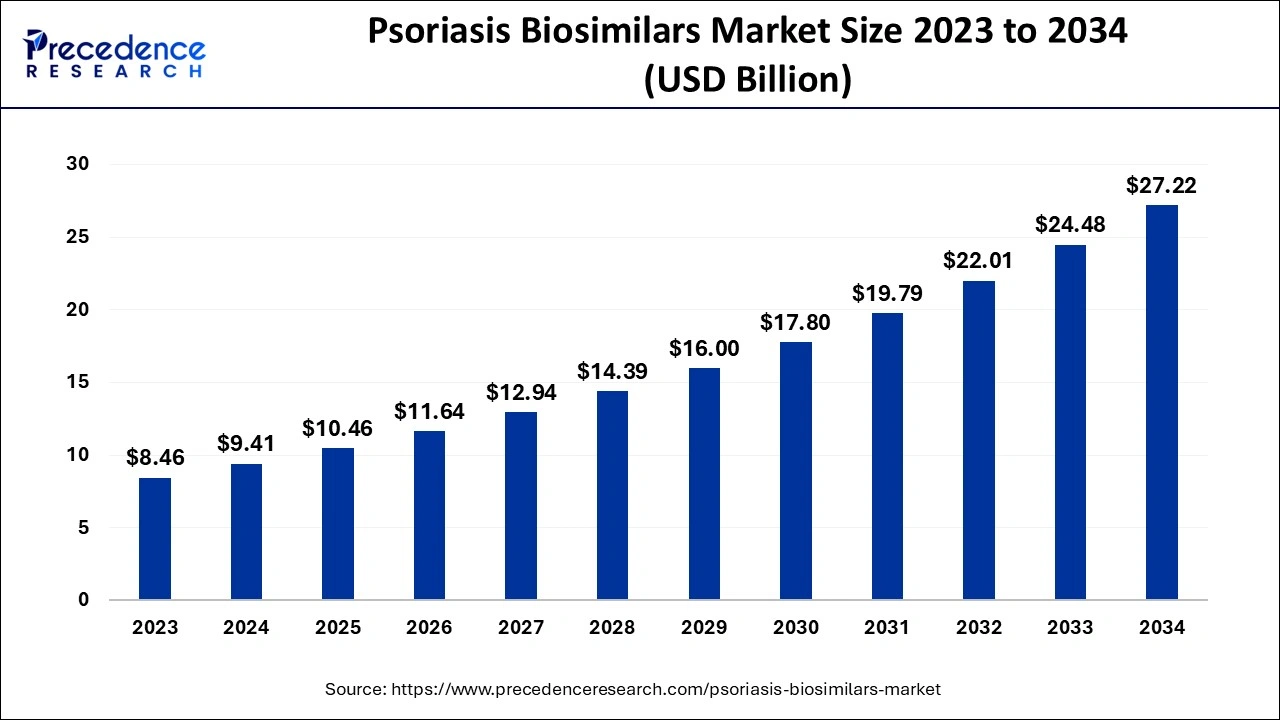
The global psoriasis biosimilars market size is calculated at USD 10.46 billion in 2025 and is projected to surpass around USD 27.22 billion by 2034, expanding at a CAGR of 11.21% from 2025 to 2034.
Market Highlights
- North America dominated the psoriasis biosimilars market in 2024.
- Asia Pacific is observed to host the fastest-growing market during the forecast period.
- By drug class, the TNF-alpha inhibitors segment dominated the market in 2024.
- By indication, the plaque psoriasis segment contributed the biggest market share of 85% in 2024.
- By indication, the psoriatic arthritis segment will witness rapid growth in the market during the forecast period.
- By route of administration, the topical segment holds the largest share of the market.
- By sales channel, the retail pharmacy chains segment accounted for the largest market share of 39% in 2024.
Market Overview
The pharmaceutical industry's division devoted to creating, manufacturing, and selling biosimilar medications to treat psoriasis is known as the psoriasis biosimilars market. Biosimilars are biologic medications that, while frequently less expensive, are very similar to previously approved reference biologics in terms of efficacy, safety, and quality. Due to the evolution of biosimilars, more patients can afford the latest biologic medicines, improving access to care in fields with limited insurance coverage.
For biopharma companies seeking to join the dermatology industry with a less expensive option to branded biologics, the psoriasis biosimilars market offers a profitable potential to increase market share while satisfying a critical medical need.
What is the Significance of AI in the Psoriasis Biosimilars Industry?
A great deal of biological data can be analyzed by AI systems to forecast which biosimilar compounds will be most effective in treating psoriasis. This speeds up identifying promising candidates for the development of biosimilars. AI-powered solutions, such as wearable technology and smartphone apps, enable psoriasis patients to be continuously monitored. This immediate data collection optimizes dosing schedules and enhances adherence to biosimilar therapy. In the psoriasis biosimilars market, by collecting and analyzing the data sets needed for regulatory submissions, artificial intelligence can help expedite the process. It helps biosimilars meet the stringent safety, effectiveness, and equivalence requirements set by originator biologics.
Psoriasis Biosimilars Market Growth Factors
- Since they are less expensive than the original biologic medications, biosimilars appeal to patients and healthcare professionals. This price decrease may make psoriasis therapy more accessible.
- The number of patients will probably rise as more people become aware of psoriasis and its available treatments. The market for psoriasis biosimilars may grow as a result.
- Increased competition from new biosimilar producers entering the market may result in further price reductions and more access to treatment choices.
- Governments and health insurance providers are more frequently adopting policies that promote biosimilars, which may promote their uptake and propel the psoriasis biosimilars market expansion.
Psoriasis Biosimilars Market Outlook:
- Industry Growth Overview: Between 2025 and 2034, this market is expected to rise significantly due to the increasing demand for cost-effective medicines coupled with surging cases of psoriasis globally.
- Major Investors: Numerous pharma brands are actively entering this market, drawn by joint ventures, R&D and business expansions. Several market players such as Samsung Bioepis Co., Ltd., Coherus BioSciences, Hoffmann-La Roche Ltd and some others have started investing rapidly for manufacturing biosimilars for treating psoriasis
- Startup Ecosystem: Various startup companies are engaged in research and development of biosimilars. The prominent startup brands dealing in psoriasis biosimilars comprises of Coherus BioSciences (USA), Bio-Thera Solutions (China), GeneSys Biologics and some others.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 10.46 Billion |
| Market Size in 2026 | USD 11.64 Billion |
| Market Size by 2034 | USD 27.22 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 11.21% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Class, Indication, Route of Administration, Sales Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Drivers
Increasing incidence of psoriasis
Rapid skin cell development that causes scaling on the skin's surface is a hallmark of psoriasis, a chronic autoimmune disease. It is frequently accompanied by itching and discomfort and might result in red regions covered in thick, silvery scales. Because biosimilars are less expensive than their reference biologics, a broader spectrum of patients can now obtain treatment. This is especially vital as the prevalence of psoriasis rises, and there is a greater need for efficient treatment solutions.
Patient demand for alternatives
Advocacy groups and patient organizations spread the word about biosimilars and other therapeutic choices. They promote dialogue between patients and medical professionals, which increases demand for substitutes. Studies have shown numerous biosimilars to be as safe and effective as their reference biologics. Due to shared experiences, demand rises when patients have favorable results with biosimilars.
Restraint
Regulatory hurdles
Biosimilars must show similarities to their reference biologics through extensive clinical research, including immunogenicity, pharmacokinetics, and pharmacodynamics evaluations. Psoriasis biosimilars market entry is delayed because these trials frequently demand a significant investment of time and money. Regulatory obstacles may cause patients and professional providers to doubt the safety and effectiveness of biosimilars compared to their reference biologics. This notion may hamper the adoption and application of biosimilars in clinical practice.
Opportunity
Rising demand for personalized medicine
Since biosimilars are less expensive than biological medicines, a larger patient base can now obtain them. This cost-cutting measure supports the customized care paradigm by removing financial obstacles to individualized treatment strategies. Doctors are becoming more and more interested in individualized treatment plans. Adoption and use can be increased by informing medical professionals about the advantages of biosimilars in providing individualized treatment. Opportunities for the psoriasis biosimilars market are presented by emerging markets' increased knowledge of personalized medicine. The demand for biosimilars may rise as these markets embrace tailored techniques more easily as healthcare infrastructure improves.
Segment Insights
Drug Class Insights
The TNF-alpha inhibitors segment dominated the psoriasis biosimilars market in 2024. The tumor necrosis factor-alpha (TNF-α) is essential to the inflammatory processes of psoriasis and is the target of TNF-alpha inhibitors. By inhibiting TNF-α, these inhibitors lessen inflammation and the skin cell proliferation that psoriasis patients experience. When the patents on initial biologics such as Enbrel and Humira expired, biosimilars appeared on the market, providing equivalent therapeutic efficacy at lower costs. The availability of TNF-alpha inhibitors has increased since the introduction of biosimilars, which have significantly reduced the price burden on individuals and healthcare systems. This price advantage reinforced the market supremacy of TNF-alpha inhibitor biosimilars in the psoriasis market.
Indication Insights
The plaque psoriasis segment led the global psoriasis biosimilars market in 2024. About 80% to 90% of all psoriasis cases globally are of the most prevalent type, plaque psoriasis. There is a great need for efficient therapies because of the high prevalence. Healthcare professionals are now more comfortable prescribing biosimilars for plaque psoriasis as clinical evidence for their safety and effectiveness has increased. Real-world data shows comparable patient results across biosimilars, and their biologic equivalents lend additional credence to this.
The psoriatic arthritis segment will witness rapid growth in the psoriasis biosimilars market during the forecast period. People with psoriasis can develop psoriatic arthritis (PsA), a chronic inflammatory disease. According to studies, up to 30% of psoriasis sufferers go on to develop psoriatic arthritis. The incidence of PsA rises in tandem with the frequency of psoriasis. Initially, physicians and patients questioned whether biosimilars were as safe and useful as the original biologics. Furthermore, it treats psoriatic arthritis and reference biologics without compromising their safety or therapeutic outcomes, according to mounting data and clinical trial studies. This has a major impact on patients' lifestyles and is directly linked to mobility issues, joint stiffness, and excruciating pain.
Route of Administration Insights
The topical segment holds the largest share of the psoriasis biosimilars market. Using topical treatments directly to the skin, psoriasis plaques can be treated locally. Compared to systemic treatments such as injections or oral drugs, which need physician monitoring or have more serious side effects, this delivery route is non-invasive and simpler for patients to employ. In general, topical biosimilars are less expensive than systemic treatments. Because biosimilars are less expensive than name-brand biologics, a broader range of patients can now obtain them.
Sales Channel Insights
The retail pharmacy chains segment held a significant share of the psoriasis biosimilars market in 2024. Retail pharmacy chains often have vast distribution networks that allow them to serve many patients in various geographic areas. This broad reach ensures that psoriasis biosimilars are easily accessible locally, increasing their accessibility for needy patients. There is a smooth supply chain for these drugs due to pharmacy chains such as CVS, Walgreens, Rite Aid, and others, which have thousands of locations around the country, some of which are international.
Regional Insights
North America dominated the psoriasis biosimilars market in 2024. Patients and healthcare professionals in North America are becoming more aware of and receptive to biosimilars. Clinicians' confidence in prescribing biosimilars has increased because of educational activities from regulatory agencies such as the FDA and biopharmaceutical companies. The usage of biosimilars to treat psoriasis has risen as medical professionals gain more details of its safety and effectiveness.
Asia Pacific is observed to host the fastest-growing psoriasis biosimilars market during the forecast period. In the past, psoriasis was frequently misdiagnosed or treated inadequately, particularly in rural or underdeveloped areas. As knowledge grows, more patients seek therapy, which increases the potential market for biosimilars. Due to their high cost, patients, particularly those in underdeveloped nations, have limited access to traditional biologics used to treat psoriasis. Biosimilars are becoming more widely used since they provide a more affordable choice. For instance, Biocon and Celltrion are leading the way in the development of biosimilars. Their ability to produce cost-effective, high-quality biosimilars has greatly aided the business's expansion.
What led Europe to hold a significant share of the psoriasis biosimilars market?
Europe held a significant share of the market. The rising cases of psoriatic arthritis in several countries such as Germany, France, Italy, UK and some others has boosted the market expansion. Additionally, rapid investment by pharma companies for opening new biosimilar research and development centers is expected to accelerate the growth of the psoriasis biosimilars market in this region.
What made Latin America to hold a considerable share of the psoriasis biosimilars market?
Latin America held a considerable share of the industry. The increasing demand for cost-effective medicines from middle-class consumers of Brazil and Argentina has driven the market expansion. Also, the rise in number of hospitals coupled with surging cases of plaque psoriasis is expected to foster the growth of the psoriasis biosimilars market in this region.
How did Middle East and Africa held a notable share of the psoriasis biosimilars market?
Middle East and Africa held a notable share of the market. The growing development in the healthcare sector in various nations including UAE, Qatar, Saudi Arabia, and some others has driven the industrial growth. Moreover, rapid investment by government for strengthening the pharma sector is expected to propel the growth of the psoriasis biosimilars market in this region.
Key Players in Psoriasis Biosimilars Market and Their Offerings
- Pfizer Inc.: Pfizer is a global biopharmaceutical company founded in 1849 that specializes in creating innovative medicines, vaccines, and consumer health products. This company has a presence in over 125 countries and applies its global resources to bring therapies to people.
- Novartis International AG: Novartis International AG is a Swiss pharmaceutical company headquartered in Basel, Switzerland, focused on discovering, developing, and manufacturing innovative medicines. It offers a wide range of products, including those for cancer, cardiovascular diseases, and neurological disorders, and provides generic medicines through its subsidiary Sandoz.
- Amgen Inc: Amgen Inc. is a multinational biotechnology company that discovers, develops, manufactures, and markets human medicines for serious diseases in areas like cancer, heart disease, and rare diseases. This company uses advanced human genetics, data science, and other technologies to develop innovative biologic therapies.
- Fresenius Kabi: Fresenius Kabi is a global healthcare company that provides medicines and technologies for the care of critically and chronically ill patients. The company focuses on four main areas: biopharmaceuticals (including biosimilars), clinical nutrition, medical technologies (such as infusion pumps and blood collection systems), and I.V. generic drugs and fluids.
- Viatris: Viatris is a global healthcare company formed in November 2020 by the combination of Pfizer's Upjohn business and Mylan. It develops, manufactures, and distributes a wide range of generic and branded medicines, biosimilars, and over-the-counter healthcare products.
- Reddy's Laboratories: Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company founded in 1984, known for its focus on affordable and innovative medicines. The company operates in key areas such as gastrointestinal, cardiovascular, and oncology, and has a global presence with a mission to accelerate access to essential treatments.
- GenScript: GenScript is a global biotechnology company that provides life science research and drug development services and products. It offers a wide range of solutions, including gene synthesis, peptide and protein services, antibody development, and cell and gene therapy manufacturing.
- Celltrion: Celltrion is a South Korean biopharmaceutical company specializing in the research, development, and manufacture of biosimilars and novel therapeutics. It pioneered the global biosimilar market and develops a range of biologics, including monoclonal antibodies for diseases such as rheumatoid arthritis and cancer.
Recent Developments
- In May 2025, Biocon Biologics, announced the approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for Yesintek. Yesintek is a biosimilar developed for treating psoriasis.
- In March 2025, Celltrion launched Steqeyma. Steqeyma is a biosimilar designed for treating psoriatic arthritis and plaque psoriasis.
- In March 2025, Fresenius Kabi launched Otulfi. Otulfi is a biosimilar designed for patients suffering from psoriasis.
Segments Covered in the Report
By Drug Class
- TNF-Alpha Inhibitors
- Infliximab
- Etanercept
- Adalimumab
- Other Biosimilars
By Indication
- Plaque Psoriasis
- Psoriatic Arthritis
- Other Types
By Route of Administration
- Subcutaneous
- Intravenous
- Oral
- Topical
By Sales Channel
- Hospital Pharmacies
- Retail Pharmacy Chains
- Online Pharmacies
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting