What is the Blockchain-Enabled Clinical Trial Integrity Market Size?
The global blockchain-enabled clinical trial integrity market involves the use of blockchain technology to secure and validate clinical trial data. It improves transparency, data integrity, and regulatory compliance in global clinical research.The market is experiencing robust growth, driven by growing regulatory scrutiny and the pursuit of decentralized and patient-focused trials.
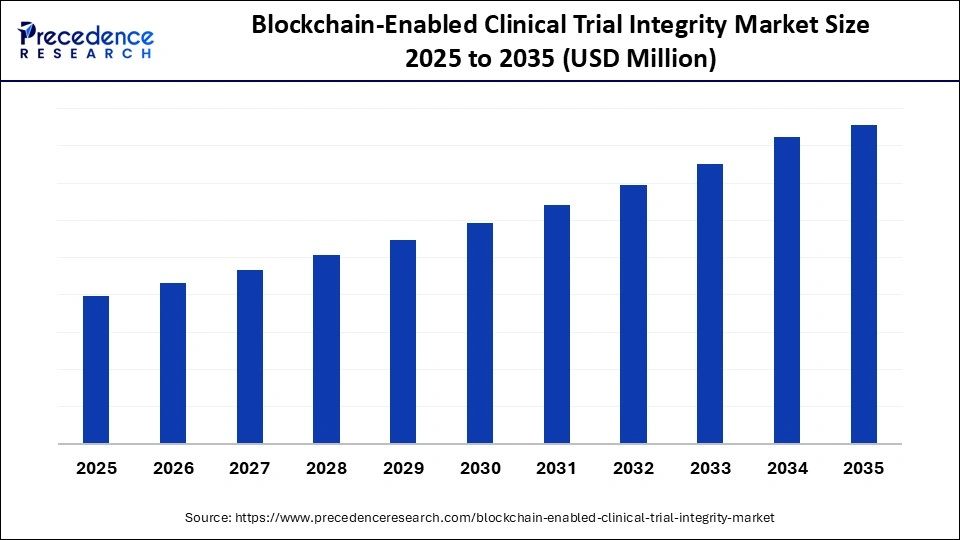
Market Highlights
- North America dominated the global blockchain-enabled clinical trial integrity market with a share of approximately 53% in 2025.
- Asia-Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
- By application, the patient consent management segment held a dominant position in the market with a share of approximately 44% in 2025.
- By application, the regulatory reporting segment is expected to grow at the fastest CAGR in the market between 2026 and 2035.
- By blockchain type, the private/permissioned segment led the global market with a share of approximately 63.5% in 2025.
- By blockchain type, the consortium blockchain segment is expected to grow with the highest CAGR in the market during the studied years.
- By molecule type, the small molecules segment dominated the global market with a share of approximately 56% in 2025.
- By molecule type, the biologics/large molecules segment is expected to expand rapidly in the market in the coming years.
- By end-user, the pharmaceutical companies segment held a major revenue share of approximately 48.5% in the market in 2025.
- By end-user, the biotechnology companies segment is expected to gain the highest share of the market between 2026 and 2035.
Blockchain Technology: Trust, Transparency, and Traceability in Trials
The blockchain-enabled clinical trial integrity market is already gaining great traction with stakeholders pursuing the impossibility of controlling trial lifecycles through the management of real-time data that is auditable and immutable. Blockchain platforms also guarantee secure consent management, data provenance, and immutable trial records. These solutions are becoming popular in pharmaceutical companies and CROs to minimize disputes, rework, and compliance risks. The global trend toward decentralized and hybrid models of clinical trials is also benefiting the market.
Role of AI in the Blockchain-Enabled Clinical Trial Integrity Market
Integrating AI into blockchain enables data validation, anomaly detection, and flagging protocol violations as they occur. Machine learning programs process clinical trial data that is recorded in blockchain ledgers to improve decision-making and predictive risk management. AI-based insights enhance patient recruitment, monitoring, and endpoint analysis, and blockchain provides data integrity. Collectively, AI and blockchain are transforming clinical trials to become more intelligent, transparent, and efficient ecosystems.
Blockchain-Enabled Clinical Trial Integrity Market Trends
- Virtual Trials: Trials leverage automation through smart contracts to streamline processes and enhance data security. This technological integration reduces errors and accelerates trial timelines.
- Rising Adoption of Advanced Technology: Pharmaceutical companies increasingly leverage blockchain technology in regular clinical trial operations, fostering innovation and resource sharing. This aims to improve trial efficiency and facilitate the adoption of new digital tools.
- Management and Data Ownership: Management and data ownership in the blockchain transformation have been enabled by introducing decentralized control and role-based access across sponsors, investigators, and regulators. Smart contracts automate permissions, workflows, and compliance checks, certifying accountability.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Application, Blockchain Type, Molecule Type, End-User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Segmental Insights
Application Insights
Which Application Segment Dominated the Blockchain-Enabled Clinical Trial Integrity Market?
The patient consent management segment dominated the market with a share of approximately 44% in 2025, driven by achieving transparency, immutability, and real-time traceability of consent records. By providing access to time-stamped consent ledger books, blockchain removes errors in consenting on a manual basis by authorized parties. This enhances trust in patients and is also in tandem with high standards of ethical and regulatory standards in clinical research. The increasing focus on patient-centric trials is another factor that increases the adoption of blockchain technology. Sponsors and CROs find blockchain increasingly helpful in streamlining the process of consent, re-consent, and updating across trial stages.
The regulatory reporting segment is expected to show the fastest growth over the forecast period, because authorities are requiring greater data integrity, audit-ready, and real-time access to trial activity. This is because blockchain simplifies regulatory filings, whereby the data about the trials and protocol change has one verifiable source of truth. Automated audit trail saves sponsors and CROs from reporting delays and compliance risks. Blockchain technology is especially useful during trials conducted across diverse geographical locations.
Blockchain Type Insights
Why Did the Private/Permissioned Segment Dominate the Market?
The private/permissioned segment held the largest revenue share of approximately 63.5% in the blockchain-enabled clinical trial integrity market in 2025, because of the sense of control, high security, and suitability of regulatory compliance. Only the authorized participants sponsor, CROs, and regulators are allowed to access and validate data within these networks. They are more advantageous than public blockchains in performance, scaling, and data privacy. Healthcare organizations are favoring permissioned systems to comply with data protection policies and internal governance policies. They are also compatible with enterprise-level IT, which also contributes to increased adoption.
The consortium blockchain segment is expected to witness the fastest growth in the market over the forecast period, since joint clinical trials are increasingly prevalent. Such platforms allow various organizations to manage and validate trial data together, and have no one controlling authority. They increase transparency and confidence among sponsors, CROs, research sites, and regulators. Large, multi-center, and global trials are most effectively done using consortium models. The adoption of this form of blockchain is rising as more industries collaborate with each other.
Molecule Type Insights
How the Small Molecules Segment Dominated the Market?
The small molecules segment contributed the biggest revenue share of approximately 56% in the blockchain-enabled clinical trial integrity market in 2025, because they have large volumes, standardized protocols, and vast development pipelines worldwide. Blockchain is used to verify data integrity throughout a complex trial stages. It assists in efficient documentation, consent, and regulatory compliance of these trials. The adoption of blockchain in this case by pharmaceutical companies is aimed at minimizing work redundancies and audit risks. The growing small-molecule R&D activities also augment the segment's growth.
The biologics/large molecules segment is expected to grow with the highest CAGR in the market during the studied years, since these trials are extremely complex and data-driven. Blockchain helps deal with difficulties in the traceability of data, cold-chain requirements, and protocol violations. The emergence of cell and gene therapies is enhancing the need for enhanced data integrity solutions. With the growth of biologics pipelines, the rapid adoption of blockchain is growing.
End-User Insights
Which End-User Segment Led the Blockchain-Enabled Clinical Trial Integrity Market?
The pharmaceutical companies segment led the market with a share of approximately 48.5% in 2025, owing to their high clinical trial activities and great emphasis on regulation. Blockchain enables companies to enhance data credibility, minimize trial delays, and increase audit preparedness. It also facilitates effective coordination in global trial locations and partners. Pharmaceutical companies increasingly invest in digital transformation. Blockchain is a risk-mitigation strategy used by large pharma players.
The biotechnology companies segment is expected to grow at the fastest CAGR in the market between 2026 and 2035, because they are carrying out more complex and risky clinical trials. Blockchain assists small biotech companies in gaining the trust of regulators and collaborators, as it is a way to provide clear data management. It eliminates reliance on manual procedures and outside audits. The shift towards novel biologics and customized treatments contributes to the uptake. The use of blockchain is rapidly increasing as the number of biotech pipelines increases.
Regional Insights
Why Did North America Dominate the Blockchain-Enabled Clinical Trial Integrity Market?
North America dominated the market with a share of approximately 53% in 2025, driven by evolving regulatory frameworks, early adoption of blockchain, and high clinical trials. The presence of major pharmaceutical firms, CROs, and technology providers promotes the adoption and use of blockchain technology. Data integrity and auditability have been highlighted by regulatory agencies. The development is also stimulated by large investments in digital health infrastructure. The region is also a leader in pilot projects and real-life blockchain applications.
Country Level Analysis
The US holds the major regional share because of the high concentrations of clinical trials, a well-developed digital health infrastructure, and the high importance of regulatory focus on data integrity. Pharmaceutical companies and CROs actively test and develop blockchain solutions to enhance audit preparedness, patient consent management, and trial monitoring in real-time.
Canada is next in gradual adoption, enabled by digital health programs of the government and increased cross-border clinical partnerships. Integration and commercialization are made faster by the existence of top-tier technology providers in the blockchain sector. The regional regulatory authorities are becoming receptive to decentralized data structures, which promotes innovation.
How is Asia-Pacific Growing in the Blockchain-Enabled Clinical Trial Integrity Market?
Asia-Pacific is expected to witness the fastest growth during the predicted timeframe, due to the growing volume of clinical trials and growing regulatory modernization. The region is adopting digital tools to enhance the efficiency and transparency of the trial process. The increase in the pharmaceutical and biotechnology R&D investments drives blockchain adoption. Governments are promoting innovations in digital health, boosting market growth. The increased involvement in global clinical trials further stimulates the growth in the region.
Country Level Analysis
China is a critical factor in the development of Asia-Pacific since the country has enhanced its pharmaceutical research and development foundation and governmental pressure to digitalize healthcare. Japan makes a big contribution to precision medicine, regulatory modernization, and secure systems of clinical data management. India has become a high-potential market that is based on the growth of clinical trial outsourcing, cost benefits, and the growing use of blockchain as a tool of transparency and compliance.
Pilot blockchain projects and favorable regulatory frameworks are also catching up in South Korea and Australia. The region has the advantage of an increase in multi-country clinical trials that need reliable data-sharing mechanisms. Asia-Pacific is the most dynamic market due to the rapid digitization of healthcare and changes in regulations.
Will Europe Grow in the Blockchain-Enabled Clinical Trial Integrity Market?
Europe is expected to grow at a notable CAGR in the foreseeable future, driven by trust, transparency, and cross-border data sharing, which are taking center stage. The multinational clinical trial environment of the region is complicated. The immutable data records are essential in the coordination and compliance of clinical trials. Good data protection standards in GDPR are compelling sponsors to implement secure permissioned blockchain systems.
Blockchain is drawing the interest of pharmaceutical companies to minimize the administrative workload and enhance trial credibility. The use of technology is being enhanced through the work of regulators, academia, and technology providers. The focus on the ethical study and patient rights is another strength of Europe in promoting the continuous growth of the market.
Country Level Analysis
Germany is a high-performing country compared to other countries in terms of its pharmaceutical manufacturing base and the early development of digital health technologies in clinical research. The UK has been proactively considering the use of blockchain in decentralized trials and regulatory reporting with the help of healthcare policies that promote innovation.
France is on the rise via public-private research partnerships on the governance of secure data. Switzerland has the world's pharma giants investing in modern trial facilities. The Nordic countries and the Netherlands are becoming pilot countries on patient-centric and data-sharing clinical models. These countries form a balanced ecosystem that contributes to the long-term and plausible growth of Europe.
Who are the Major Players in the Global Blockchain-Enabled Clinical Trial Integrity Market?
The major players in the blockchain-enabled clinical trial integrity market include IBM Life Sciences, Oracle Health, Microsoft (Azure Blockchain), Guardtime, Embleema, Chronicled BurstIQ, Patientory, SGS SA , Eurofins Scientific, Clinical6 (Excellis, ConsenSys, Trial, Medicalchain, Hashed Health
Recent Developments
- In November 2025, NTU Singapore and Zero Gravity (0G) announced a $5 million partnership to establish a joint research hub advancing blockchain-based AI technologies, enhancing accessibility and accountability. The initiative aims to bridge academic research and scalable infrastructure for open AI systems.(Source: https://www.ntu.edu.sg)
- In March 2025, ConcertAI collaborated with NVIDIA to build multi-modal oncology data with an integrated network of specialized AI agents, including LLMs, SLMs, and advanced. These agents aim to streamline clinical trial workflows by analyzing data from tens of thousands of cancer patients, rapidly spotting treatment patterns, and suggesting optimal clinical trial designs. (Source: https://www.drugdiscoverytrends.com)
Segments Covered in the Report
By Application
- Patient Consent Management
- Clinical Trial Data Management
- Recruitment & Retention
- Regulatory Reporting
By Blockchain Type
- Private/Permissioned Blockchain
- Public Blockchain
- Consortium Blockchain
By Molecule Type
- Small Molecules
- Biologics/Large Molecules
By End-User
- Pharmaceutical Companies
- Biotechnology Companies
- CROs & Research Institutes
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting