Medical Devices Vigilance Market Size and Forecast 2025 to 2034
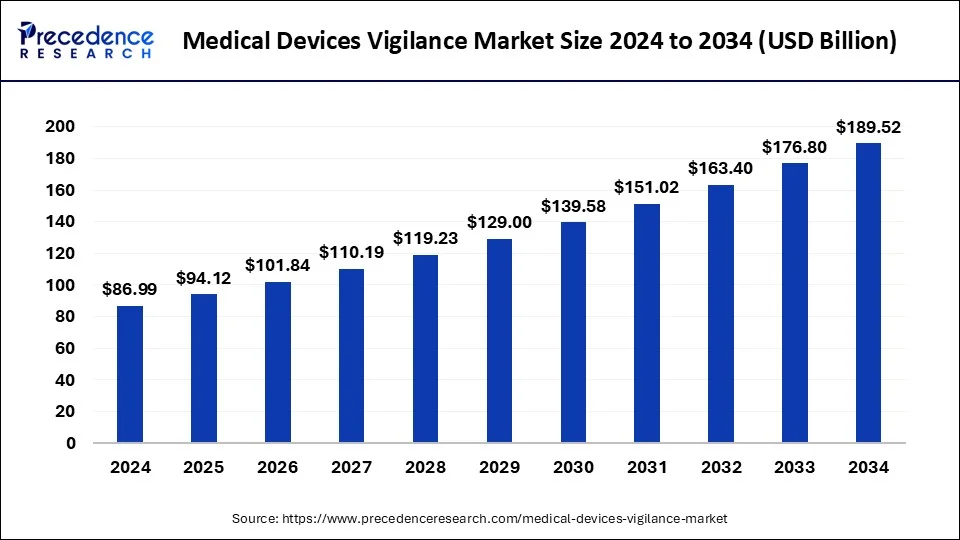
The global medical devices vigilance market size was USD 86.99 billion in 2024, estimated at USD 94.12 billion in 2025 and is anticipated to reach around USD 189.52 billion by 2034, expanding at a CAGR of 8.10% from 2025 to 2034. Instances of device failures prompting investment in vigilance systems to prevent future incidents with the safety of patients is the prime purpose of the healthcare system.
Medical Devices Vigilance Market Key Takeaways
- The global medical devices vigilance market was valued at USD 86.99 billion in 2024.
- It is projected to reach USD 189.52 billion by 2034.
- The medical devices vigilance market is expected to grow at a CAGR of 8.10% from 2025 to 2034.
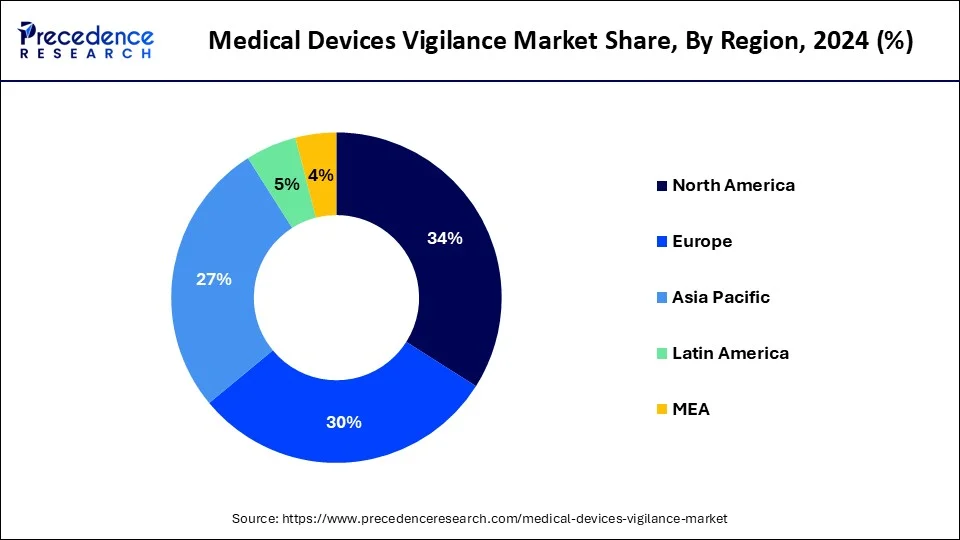
- North America dominated the global market with the largest market share of 34% in 2024
- Asia Pacific is projected to witness rapid growth during the forecast period.
- By delivery mode, the on-demand segment contributed the highest market share of 81% in 2024.
- By delivery mode, the on-premises segment is expected to have steady growth over the forecast period.
- By application, the diagnostics segment held the largest market share of 36% in 2024.
- By application, the research segment is expected to show lucrative growth over the forecast period.
- By end use, the clinical research organization segment held the highest market share of 42% in 2024.
- By end use, the business process outsourcing firms segment is expected to grow rapidly in the foreseeable period.
U.S. Medical Devices Vigilance Market Size and Growth 2025 to2034
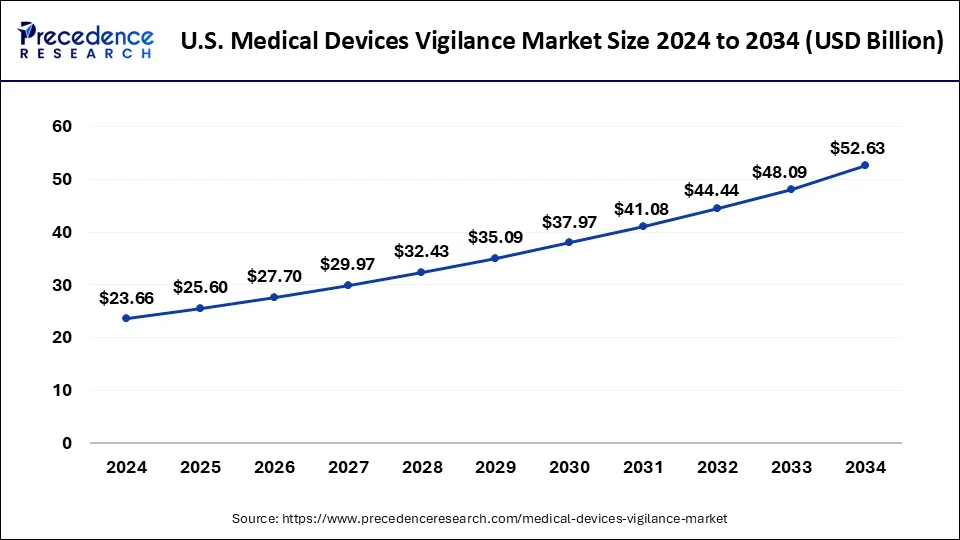
The U.S. medical devices vigilance market size surpassed USD 23.66 billion in 2024 and is expected to be worth around USD 52.63 billion by 2034 at a CAGR of 8.32% from 2025 to 2034.
North America is estimated to hold a substantial share of the medical devices vigilance market. It represents a lucrative market for medical device vigilance, characterized by a strong regulatory environment, high adoption of medical devices, and a culture of technological innovation, driving continued growth and advancement in the field.
In North America, the medical devices vigilance market is poised for significant growth due to several factors. First, the region boasts a well-established healthcare infrastructure with advanced medical facilities and a high adoption rate of medical devices. This widespread use of medical devices, ranging from implantable devices to diagnostic equipment, creates a robust demand for vigilance systems to ensure patient safety. Secondly, stringent regulatory frameworks in countries like the United States and Canada mandate deep monitoring and reporting of adverse events associated with medical devices.
Furthermore, the North American region is at the forefront of technological innovation, with a growing emphasis on leveraging artificial intelligence, machine learning, and big data analytics in healthcare. This presents opportunities for companies in the medical devices vigilance market to develop cutting-edge solutions that harness these technologies to enhance safety and efficiency.
Asia Pacific is the fastest-growing region of the global medical devices vigilance market, and its rapid growth is fuelled by several key factors. The foremost factor is the increasing demand for healthcare services in high-population countries such as China, India, and Japan, which is driving the adoption of medical devices across various specialties.
As the use of medical devices expands, there is a growing awareness of the importance of ensuring their safety and effectiveness, leading to heightened demand for vigilance solutions. Besides, regulatory bodies in the region are increasingly focusing on strengthening regulations related to medical device safety and surveillance. Countries like China and India are enhancing their regulatory frameworks to align with international standards, mandating stricter monitoring and reporting requirements for adverse events associated with medical devices. This regulatory push is driving the need for robust vigilance systems and creating opportunities for companies offering these kinds of solutions.
- India has launched a program popularly known as the Materiovigilance Programme of India (MvPI). MvPI aims to collect data on medical device-related adverse events systematically and scientifically analyze them to aid in regulatory decisions and recommendations on the safe use of medical devices.
Additionally, Asia Pacific is witnessing rapid technological advancements and digitalization in healthcare, providing a fertile ground for innovation in medical device vigilance. Companies are leveraging technologies such as artificial intelligence, big data analytics, and cloud computing to develop sophisticated vigilance solutions that can efficiently monitor device performance and identify potential risks. Therefore, the region presents a dynamic and rapidly growing market for medical device vigilance, driven by increasing healthcare demand, evolving regulatory landscapes, and technological advancements. This market offers significant opportunities for growth and investment.
Market Overview
The medical devices vigilance market keeps an eye on the safety of medical tools and equipment. It's like a watchdog, making sure everything works as it should and doesn't harm people. It basically works as a security system for medical devices. The market is growing because more devices are being used, like pacemakers and insulin pumps.
With more devices, there's a greater need to make sure they're safe and effective. For instance, if a pacemaker suddenly stops working, it could be dangerous. So, companies are investing in vigilance systems to prevent problems like this. Overall, the market for medical device vigilance is expanding because people want to feel confident that the devices rely on medical devices that are safe and reliable for medical intervention as it might be risky and life-threatening if done wrongly, even by professionals or less skilled physicians. Thus, medical device vigilance highlights the importance of the safety of patients and, eventually, drives the growth of the market rapidly.
Medical Devices Vigilance Market Growth Factors
- Increasing use of medical devices such as pacemakers and insulin pumps.
- Growing awareness about the importance of device safety and its proper uses, including keeping an eye on it.
- Strict regulatory requirements pushing for stricter vigilance measures.
- Advancements in technology make it easier to monitor device performance.
- Rising demand for quality healthcare services driving the need for reliable devices.
- Instances of device failures prompting investment in vigilance systems to prevent future incidents.
Market Scope
| Report Coverage | Details |
| Global Market Size in 2024 | USD 86.99 Billion |
| Global Market Size in 2025 | USD 94.12 Billion |
| Global Market Size by 2034 | USD 189.52 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.10% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Delivery Mode, Application, End-user, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Medical Devices Vigilance Market Dynamics
Driver
Importance of monitoring
Increasing reliance on medical devices worldwide. As technology advances, more sophisticated devices are developed to diagnose, treat, and monitor various medical conditions. For instance, pacemakers, insulin pumps, and implantable defibrillators have become essential for managing cardiac issues, while continuous glucose monitors are crucial for diabetic patients.
Still, with the proliferation of these devices, there's a heightened concern about their safety and effectiveness. Instances of device malfunctions or failures can have serious consequences for patients, including injury or even death. As a result, regulatory bodies are imposing stricter requirements for the monitoring and reporting of adverse events associated with medical devices.
In the United States, a recent study published in JAMA Internal Medicine reported that 23% of events reported as serious injury or death were not correctly attributed to a defective medical device and were reported incorrectly. This was equivalent to about 31,500 deaths caused by unsafe medical devices, which were potentially preventable through an appropriate system of corrective and preventive actions.
Additionally, healthcare providers and consumers are becoming more aware of the importance of device vigilance in ensuring patient safety. They are increasingly demanding assurances that the devices they use are not only effective but also reliable and free from defects. Consequently, companies in the medical devices vigilance market are experiencing growing demand for their services and solutions to address these concerns and comply with regulatory standards. Thus, safety concerns with sophisticated medical equipment become a booming factor in the medical devices vigilance market on a global scale.
Restraint
Complexity and cost
A major restraint for the medical devices vigilance market is the complexity and cost associated with implementing vigilance systems. Developing and maintaining robust systems for monitoring, reporting, and analyzing adverse events require significant investment in technology, infrastructure, and skilled personnel. Furthermore, the regulatory landscape governing medical devices is continuously evolving, with stringent requirements for compliance. Navigating these regulations and following a stringent rule for reporting obligations can be challenging for companies, particularly smaller enterprises with limited resources.
Moreover, there may be resistance from some stakeholders within the healthcare industry who perceive vigilance measures as a burden or disruptive to their operations. This resistance can hinder the widespread adoption of vigilance practices and delay the implementation of necessary improvements to device safety protocols. Thus, the demand for vigilance solutions is increasing, but these barriers may slow down the pace of growth in the medical devices vigilance market and pose challenges for companies aiming to expand their presence in this sector.
Opportunity
integration of advanced technologies
A significant future opportunity for the medical devices vigilance market lies in the integration of advanced technologies such as artificial intelligence (AI) and machine learning (ML). By leveraging AI and ML algorithms, vigilance systems can more effectively identify patterns and trends within large datasets of adverse event reports. It aids in enabling early detection of potential safety issues. This proactive approach allows manufacturers to take timely corrective actions to prevent harm to patients and minimize potential risks.
Besides, AI-powered predictive analytics can enhance risk assessment and enable personalized risk management strategies based on patient-specific factors such as medical history, region where he lives, and genetic factors running in the hierarchy of the family he belongs to. This personalized approach not only improves patient safety but also enhances overall healthcare outcomes.
Moreover, the integration of AI and ML can streamline regulatory compliance processes by automating documentation, reporting, and audit management, thereby reducing companies' administrative burdens and costs. Hence, the adoption of advanced technologies presents a promising opportunity for the medical devices vigilance market to enhance safety, improve efficiency, and drive innovation in the healthcare industry.
Delivery Mode Insights
The on-demand segment accounted for the largest share of the market in 2024. The on-demand delivery segment in the medical devices vigilance market ensures swift access to crucial medical devices. This service prioritizes timely delivery, catering to urgent medical needs and emergencies. By leveraging technology and efficient logistics, it streamlines the distribution process, ensuring seamless access to medical devices when and where they are needed most. This segment plays a vital role in enhancing patient care and safety by reducing response times and ensuring a continuous supply of essential medical equipment.
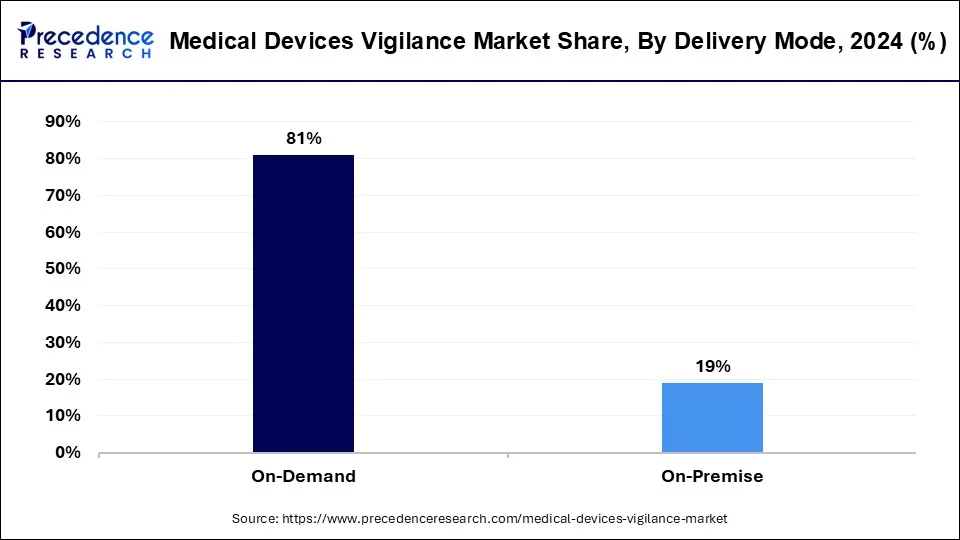
The on-premises segment has generated a 19% revenue share in 2024. and is expected to have steady growth over the forecast period. The on-premises mode segment in the medical devices vigilance market offers a localized approach to device management. It enables healthcare facilities to maintain control over the vigilance process by managing it within their own premises. This segment provides the flexibility to customize vigilance protocols according to specific needs and regulations. By keeping vigilance activities in-house, healthcare providers can ensure greater confidentiality and compliance with data protection laws. Additionally, it allows for immediate response to incidents. And promote prompt resolution and patient safety.
Application Insights
The diagnostics segment held a substantial market share in 2024. In the medical devices vigilance market, the diagnostic segment serves as a critical application. It utilizes advanced technologies to analyze and promptly detect potential issues or malfunctions in medical devices. By employing diagnostic tools and algorithms, this segment can assess device performance, identify anomalies, and alert healthcare providers to take necessary actions. This proactive approach helps prevent adverse events, ensures device efficacy, and enhances patient safety. Through continuous monitoring and analysis, the diagnostic segment contributes to maintaining the integrity and reliability of medical devices in the healthcare ecosystem.
The research segment is expected to show lucrative growth over the forecast period. The research segment serves as a pivotal application, focusing on advancing knowledge and understanding of device safety and performance. Researchers investigate trends, analyze data, and evaluate the effectiveness of devices in real-world settings. They conduct clinical trials, observational studies, and post-market surveillance to identify potential risks and benefits associated with medical devices. Through their findings, they contribute valuable insights to regulatory agencies, manufacturers, and healthcare providers. Thus, the research segment plays a crucial role in driving innovation and improving the safety and efficacy of medical devices.
End-use Insights
The clinical research organization segment held the highest market share in 2024. In the medical devices vigilance market, clinical research organizations (CROs) specialize in conducting clinical trials and research studies on behalf of medical device manufacturers and regulatory bodies. Their expertise in data collection, analysis, and reporting makes them instrumental in monitoring device safety and efficacy throughout the product lifecycle. By partnering with CROs, manufacturers can ensure compliance with regulatory requirements and expedite the development and commercialization of new devices. With their extensive infrastructure and experienced personnel, CROs play a dominant role in advancing medical device vigilance and driving innovation in the industry.
The business process outsourcing firms segment is expected to grow rapidly in the foreseeable period. In the medical devices vigilance market, the business process outsourced (BPO) segment emerges as a rapidly growing end-use category. BPO firms specialize in providing outsourced services for vigilance activities, such as adverse event reporting, complaint handling, and regulatory compliance. By outsourcing these tasks to specialized service providers, medical device companies can streamline their operations, reduce costs, and access expertise not available in-house.
The BPO segment offers scalable solutions tailored to the needs of device manufacturers, enabling them to focus on core competencies while ensuring compliance with regulatory requirements. With increasing demand for efficient vigilance processes, the BPO segment is poised for continued growth and expansion in the market.
Medical Devices Vigilance Market Companies
- ZEINCRO
- AssurX Inc.
- Sparta System
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- MDI Consultants, Inc.
- AB-Cube
- Laerdal Medical.
- Omnify Software, Inc.
Recent Developments
- In June 2022, Italy instituted substantial changes in national regulations on medical device vigilance in accordance with the procedures by European Regulations. 2017/475 for medical devices and 2017/476 for in vitro diagnostics.
Segments Covered in the Report
By Delivery Mode
- On-demand
- On-premise
By Application
- Diagnostics
- Therapeutics
- Surgical
- Research
By End-user
- Clinical Research Organizations (CROs)
- Business Process Outsourcing (BPO)
- Original Equipment Manufacturers (OEM)
- Other End-users
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting