What is the Neuroendocrine Tumor Treatment Market Size?
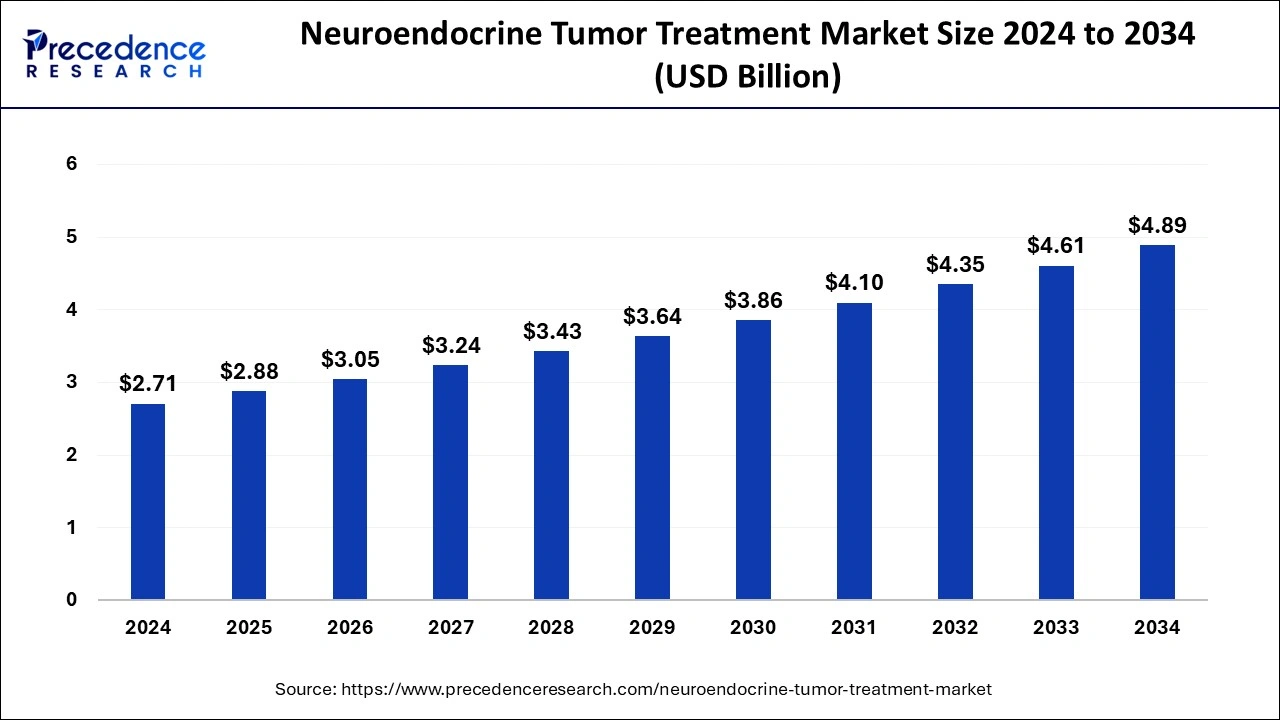
The global neuroendocrine tumor treatment market size is valued at USD 2.88 billion in 2025 and is predicted to increase from USD 3.05 billion in 2026 to approximately USD 4.89 billion by 2034, growing at a CAGR of 6.09% from 2025 to 2034. Growing awareness of neuroendocrine tumors and the importance of early detection are driving the growth of the neuroendocrine tumor treatment market. The growing demand for personalized medicines is contributing to the market expansions. Moreover, growing research and development activities are likely to boost the market expansion in the forecast period.
Neuroendocrine Tumor Treatment Market Key Takeaways
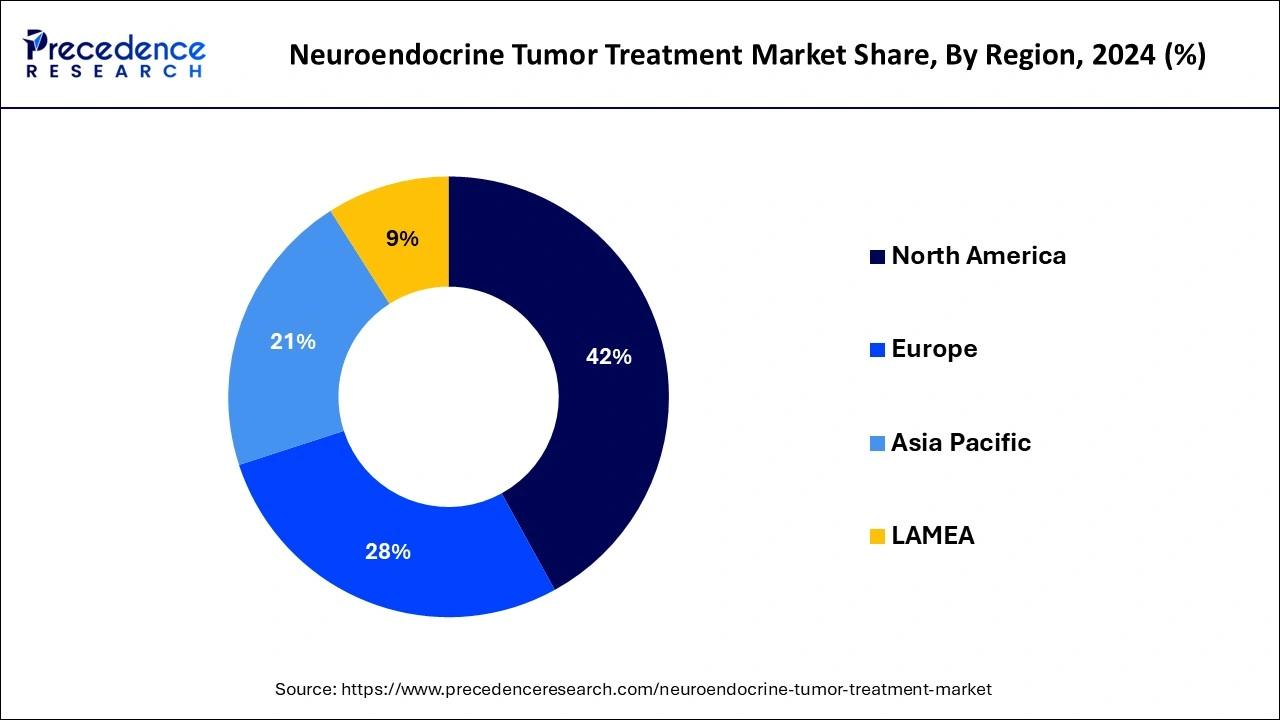
- North America dominated the global neuroendocrine tumor treatment market with the largest market share of 42% in 2024.
- Middle East and Africa is expanding at a double-digit CAGR of 11.2% during the forecast period.
- By type, the carcinoid tumors segment captured the biggest market share of 24% in 2024.
- By type, the paraganglioma segment is projected to grow at a CAGR of 7.72% during the forecast period.
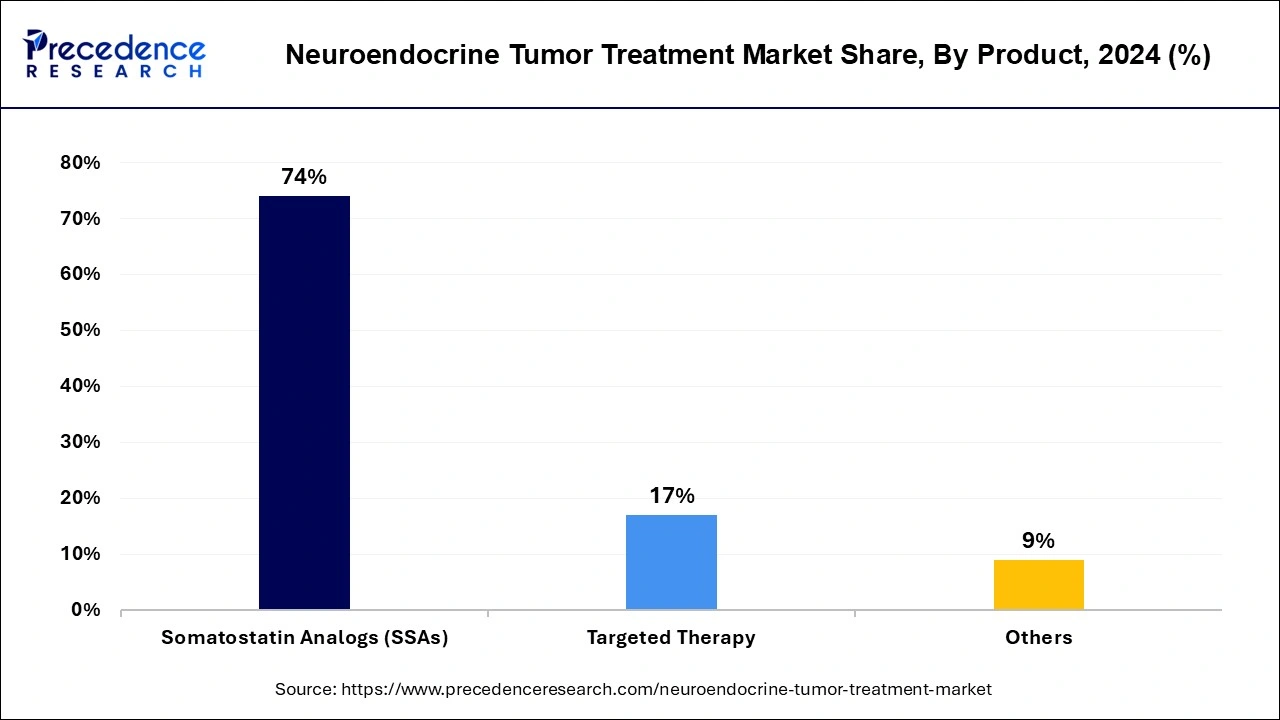
- By product, the somatostatin analogs (SSAs) segment contributed the highest market share of 74% in 2024.
- By product, the targeted therapy segment is anticipated to witness the fastest CAGR during the forecasted years.
- By site, the small intestine segment generated the major market share in 2024.
- By site, the stomach segment is expected to grow at a significant CAGR during the forecast period.
- By end-use, the hospitals segment accounted for the largest market share in 2024.
- By end-use, the clinics segment is expected to show the fastest growth during the predicted timeframe,
Artificial intelligence (AI) advantages for growth of the neuroendocrine tumor treatment market
The integration of artificial intelligence is significantly transforming the neuroendocrine tumor treatment market by enhancing diagnostic accuracy, personalized medicine plans tailored to the patient, and streamlined clinical trials. AI is enabled to identify suitable candidates for clinical trials, as well as optimize trial design and result analysis to improve efficiency. AI-enabled chatbots and VAs help to minimize burdens and allow healthcare professionals to concentrate on critical tasks. The ability of AI to predict treatment response is helping to generate targeted and effective treatment solutions.
AI also helps to identify effective treatment options for certain patients to improve patient outcomes and quality of life, which makes it easier for healthcare professionals. Additionally, AI is able to identify possible risks, which helps with risk management during trials as well as diagnostics. AI plays a crucial role in surgeries and finding rare diseases. The integration of AI is overall shifting the neuroendocrine tumor treatment market toward success by enhancing treatment, diagnosis, and patient outcomes.
- In November 2024, UC San Francisco and the University of Michigan developed an artificial intelligence (AI)-powered diagnostic tool that helps neurosurgeons to identify invisible cancer spread near the surgery area in 10 seconds. This technique is able to delay the recurrence of high-grade tumors and prevent it in lower-grade tumors.
Market Overview
The neuroendocrine tumor (NET) is a rare cancer that occurs due to abnormal growth of cells in the neuroendocrine system. The growing prevalence of neuroendocrine carcinoma and growing awareness of the NETs are the key factors expanding the growth of neuroendocrine tumor treatments. NETs are very common in older populations; also, changing lifestyles and environmental issues are causing incidences of neuroendocrine tumors. The awareness of prevalence and early detection has risen in recent years, which has led to the development of innovative therapy options. Additionally, pharmaceutical companies and governments worldwide have enhanced investments in the research and development sector for modern and effective treatment solutions.
The developments of novel medications to identify various types of carcinoid tumors and target therapies, as well as immunotherapy, are shifting the neuroendocrine tumor treatment market rapidly toward success. Additionally, the development of novel, innovative radioligand therapy is emerging in the market. The adoption of new medications, like imaging techniques, biomarkers, target therapies, and precision medicines, is crucial for improving the market conditions. The growing partnerships of academies and pharmaceutical companies are seeking new ways to improve market expansions by working together in research and clinical trials. Additionally, the market is expected to witness significant growth in the forecast period due to increasing sustainability trends and regulatory support.
- In September 2024, Abdera Therapeutics, Inc. received FDA approval for orphan drug designation for its ABD-147, a next-generation precision radiopharmaceutical biologic therapy. FDA has granted ABD-147 for use as a potential therapeutic option in patients with neuroendocrine carcinoma.
Technology Advancement
The neuroendocrine tumor treatment market makes it easier for doctors to find the disease early and treat patients in a more precise way. With the help of these tools, doctors can find out about neuroendocrine tumors (NETs) earlier and make treatment plans that are most suitable for each person who has the disease.
The development of targeted therapies, as well as Peptide Receptor Radionuclide Therapy (PRRT), has made a huge impact on therapeutic advancement. PRRT directs radiation to tumor cells by using radiolabeled somatostatin analogs that attach to those cells' receptors, protecting healthy tissue. Advancements in molecular biology have allowed for the growth of new targeted agents, such as mTOR inhibitors and tyrosine kinase inhibitors, designed to block tumor growth.
What Factors Are Contributing to the Expected Growth of the Neuroendocrine Tumor Treatment Market In 2025?
The neuroendocrine tumor (NET) treatment market is expanding rapidly, based on its global incidence, growth in targeted therapies and early diagnosis, improvement in awareness for rare cancers, and an influx of personalized medicine. The pharmaceutical sector is continuing to invest in new somatostatin analogs, peptide receptor radio-nucleotide therapies (PRRTs), and immunotherapies that improve overall survival and access for patients with NETs.
Key Factors Influencing Future Market Trends
- Rising Incidence of Neuroendocrine Tumors: Market growth mostly owes to more people getting diagnosed with neuroendocrine tumors around the world. New technologies for looking at the body, doctors, and patients talking more about medical results are helping find cancers earlier.
- Advancements in Targeted Therapies: These treatments are more precise and tend to cause fewer side effects. Doctors are starting to use them more often when treating patients. Advances in making and using drugs that go directly to a certain type of cell are making treatment better and keeping the NET treatment market growing.
- Rising Research and Development funding: There is a strong financial commitment by companies and research organizations towards finding better ways to treat NETs. The goal of these efforts is to introduce more effective and reliable treatments that will help the medical industry grow and foster new ways of treating and detecting diseases.
- Supportive Government and Regulatory Initiatives: New NET therapies are being supported by governments and regulatory agencies, which provide funding, fast approvals, and orphan drug labels. They help reduce the effort and costs involved in getting new therapies to the public.
Neuroendocrine Tumor Treatment Market Growth Factors
- Prevalence of neuroendocrine carcinoma: The changing lifestyle and environmental factors are increasing the prevalence of neuroendocrine carcinoma. The disease is largely witnessed among the aging population, leading to increased demands for neuroendocrine tumor treatments.
- Growing awareness: The awareness of neuroendocrine tumor disease has increased in recent years. Additionally, early detection and diagnostics are important to driving market growth.
- Demand for personalized medicines: The increased demand for personalized medicines are encouraging developments of innovative target therapies and precision medicines.
- Government initiatives: The growing government initiatives for research and development in the rare disease sector, like cancer research, are spectacularly fueling the market growth.
- Technology advancements: The adoption of advanced technologies like 3D scanners, imaging tests, and endoscopy are showing ways of success in the market.
- Adoption of immunotherapies: The adoption of immunotherapies like immune checkpoint inhibitors has increased, which is leading to driving the market growth.
Market Outlook
The prognosis for the NET treatment industry looks positive with the increased adoption of targeted and combination therapies. As clinical trials become more successful and innovation in nuclear medicine continues, forecasts anticipate growth in the NET therapy market through 2030. Collaborations between international and domestic companies will create manufacturing affordability and access in healthcare systems of emerging economies.
- Industry Expansion: The neuroendocrine tumor treatment industry anticipates robust CAGR growth stemming from a rising prevalence, use of earlier diagnostics, and an increasing trend towards adopting precision oncology. The sector will have fairly consistent long-term growth from ongoing R&D paired with supportive healthcare policies.
- Sustainable Practices: Sustainability in the NET treatment industry centers around eco-friendly manufacturing practices, adherence to ethical conduct in clinical trials, and reducing waste created from the use of medicines. Sustainable practices will mean that pharmaceutical companies utilize green chemistry and recyclable packaging.
- Globalization of Industrial Practices: Major industry players are expanding geographically into the Asia Pacific Region, Latin America, and the Middle East with strategic alliances and distribution partnerships. The ability to create a continentally based service will enhance access to next generation therapies and healthcare in general.
- Startup Ecosystem: Biotech startups are achieving momentum in the Net landscape, innovating new pathways for AI, genomics, and precision drug delivery systems. The improvements created by smaller innovators are accomplished via faster diagnostics, more precise treatments, and/or more effective patient monitoring to be integrated globally.
Market Scope
| Report Coverage | Details |
| Market Size by 2026 | USD 3.05 Billion |
| Market Size in 2025 | USD 2.88 Billion |
| Market Size in 2034 | USD 4.89 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.09% |
| Leading Region | North America |
| Fastest Growing Region | Middle East and Africa |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Product, Site, End-use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Increased prevalence of neuroendocrine tumor diseases
The prevalence of neuroendocrine tumors has increased in recent years due to changing lifestyles and environmental impacts. Growing obesity and physical inactivity are leading to the prevalence of the disease; also, the diseases are common in the aging population. The diagnosis of NET patients is mostly done in stage 4, making it riskier and more challenging. This complexity in neuroendocrine tumors is driving the importance of research and development in the neuroendocrine tumor treatment market. However, advanced diagnostic techniques like computed tomography (CT scans), PET scans, and magnetic resonance imaging (MRI) allow for the early detection of such and more accurate diagnoses for the NETs. Moreover, the adoption of endoscopy provides improved diagnostic treatments for NETs.
Additionally, increased awareness of neuroendocrine tumors and the importance of early detection are encouraging developments of cutting-edge diagnoses and treatment solutions. The increased prevalence of NETs has led to the adoption of innovative therapies like targeted therapies, immunotherapies, and combination therapies that are fueling the neuroendocrine tumor treatment market expansions. Moreover, the increased reporting of the NET cases is expected to attract more attention from government and regulatory bodies to invest in innovation and development firms that will help the market for further growth.
Restraints
High cost and complexity
The neuroendocrine tumor treatment market services are expensive, which is pressurizing for the patients. The cost of pharmaceutical manufacturing, as well as the development of new therapies, are impacting overall treatment costs. NETs have complex biology and clinical behavior that is challenging in the development of effective and targeted treatment solutions.
Lack of awareness and regulatory challenges
The awareness of neuroendocrine is very low among health professionals and patients; mostly, patients are taking treatments at stage 4, which leads to an unliveable solution that directly hinders the neuroendocrine tumor treatment market expansion. The stringent regulatory requirements for approvals and high costs associated with clinical trials are hampering the research and development sector's growth for neuroendocrine tumor treatments.
Limited diagnostic accuracy
The lack of specific biomarkers is making it difficult to provide accurate diagnoses for NETs due to limited access to track disease progressions and predictions for required treatment options.
Opportunity
Technology advancements
The developments of advanced treatment methodologies are projected to boost the neuroendocrine tumor treatment market expansion in the forecast period. The adoption of cutting-edge treatments, such as target therapies like somatostatin analogs and tyrosine kinase inhibitors, is enhancing patient outcomes. Similarly, personalized medicines have been popular with target therapies and precision medicine demands. Moreover, technological developments in genomics, as well as molecular biology, are allowing an improved understanding of the biological mechanisms of NETs, making it easier to generate targeted therapies.
Diagnostic technologies like CT scans, PET scans, and MRIs have been improved to provide more accurate and early diagnoses for the neuroendocrine tumor treatment market. The increased prevalence of NETs is driving the importance of early detection and diagnosis, leading to developments as well as options for advanced technologies by healthcare professionals. Government and regulatory initiatives are also contributing to these developments. Additionally, pharmaceutical companies have increased investments in research and development for innovative treatment options and expanded the treatment methodologies for neuroendocrine tumors.
Type Insights
The carcinoid tumors segment captured the biggest neuroendocrine tumor treatment market share in 2024 due to the increased incidence of carcinoid tumors. The segment has witnessed growth due to increased awareness of early diagnosis and demand for improved diagnostic techniques. The availability of advanced treatment options for carcinoid tumors, like chemotherapy, targeted therapies, and somatostatin analogs, is also helping the segment to grow. Ongoing research with vast clinical trials investigating new treatments and combination therapies, as well as a focus on advancing immunotherapies and target therapies, are driving development for carcinoid tumors.
On the other hand, the paraganglioma segment will show notable growth in the neuroendocrine tumor treatment market during the forecast period. Paragangliomas are rare and require specific treatment approaches, including radiation therapy, systemic therapies, and surgery. The increased focus of researchers on enhancing target therapies like tyrosine kinase inhibitors to treat paraganglioma is the key factor driving the growth of this segment. Increased incidence of paraganglioma is driving awareness for earlier diagnosis and treatments and increasing demand for advanced and effective treatment options. Continuous research initiatives to improve treatment solutions for paraganglioma.
Product Insights
The somatostatin analogs (SSAs) segment contributed the highest neuroendocrine tumor treatment market share in 2024 during the forecasted years with its proven record of efficacy and safety. The somatostatin analog has become the first-line treatment option for NETs like carcinoid tumors. SSAs have gained approval for treatments of several NETs, like carcinoid tumors, pancreatic NETs, and gastrointestinal NETs. SSAs are available in a wide range of dosing options, including oral and injectable, which are convenient and comfortable for patients, especially aging populations. The ongoing research for the use of SSAs in lung NETs and thyroid NETs.
- In January 2024, Novartis published the data from the Phase III NETTER-2 trial at the 2024 American Society of Clinical Oncology (ASCO) Gastrointestinal (GI) Cancers Symposium that shows long-acting release (LAR) octreotide is able to minimize the risk of disease progressions or death by 72% as first-line therapy in patients with somatostatin receptor-positive well-differentiated grade 2/3 advanced gastroenteropancreatic neuroendocrine tumors, by just using high-dose octreotide LAR.
However, the targeted therapy segment is anticipated to witness the fastest growth in the neuroendocrine tumor treatment market during the forecasted years due to its improved effectiveness in the treatment of various NETs, including pancreatic and gastrointestinal NETs. The increased focus on personalized medicines for specific tumors is rapidly expanding the segment expansion. Additionally, the ability of target therapies to deliver enhanced treatment outcomes and reduce side effects is making them preferred by healthcare professionals, with ongoing research and developments for continuous advancements and a growing need for effective treatment options.
Site Insights
The small intestine segment generated the major share in the neuroendocrine tumor treatment market in 2024. Small intestines are the common site for NETs; the major incidence of NETs occurs in small intestines. The need for specialized treatment options for small intestine NETs has increased focus on research and developments of novel, cutting-edge treatment solutions that are responsible for fueling market growth. Additionally, the availability of advanced treatment options like somatostatin analogs, targeted therapies, and chemotherapy are contributing to segment expansions. The ongoing developments of peptide receptor radionuclide therapy for small intestine NETs.
On the other hand, the stomach segment is expected to witness significant growth in the neuroendocrine tumor treatment market over the forecast period due to increased gastric NET incidence. The increased incidence of gastric NETs is driving demand for novel, cutting-edge treatment options like endoscopic resection, surgery, and somatostatin analogs. The increased awareness of stomach-related NETs among healthcare professionals and patients is fueling the segment expansions. Additionally, increased investments in developing new treatment options for gastric NETs.
End-use Insights
The hospitals segment accounted for the largest share of the neuroendocrine tumor treatment market in 2024. The segment growth is driven by the availability of well-established treatment care in settings for NET patients in the hospitals. Increased incidence of NETs has increased hospitalization; also, the ability to provide a large volume of NET treatments is driving the making of hospitals dominating the market. Hospitals provide multidisciplinary care teams for oncology, radiology, surgery, and endocrinology, which monitor and provide management for the NETs. Access to advanced diagnostic technologies like CT scans and PET makes hospitals prior to getting treatment forms. Moreover, the availability of cutting-edge treatment technologies like peptide receptor radionuclide therapy (PRRT) and stereotactic body radiation therapy (SBRT) are also contributing to the segment's growth. Growing government and non-government organizations' focus and funding to the hospitals are making easy access to advanced therapies and rising patient outcomes.
However, the clinics segment is expected to show the fastest growth in the neuroendocrine tumor treatment market during the predicted timeframe due to increased demands for specialized care. The segment growth is also driven by the providing of multidisciplinary services that bring together professionals from different fields of oncology, surgery, and endocrinology. The rising need for personalized and patient-centered care is rapidly shifting preference toward clinics. The convenient locations and access for advanced and quick treatments are projected to enhance patient preference for the clinics.
Regional Insights
U.S. Neuroendocrine Tumor Treatment Market Size and Growth 2025 to 2034
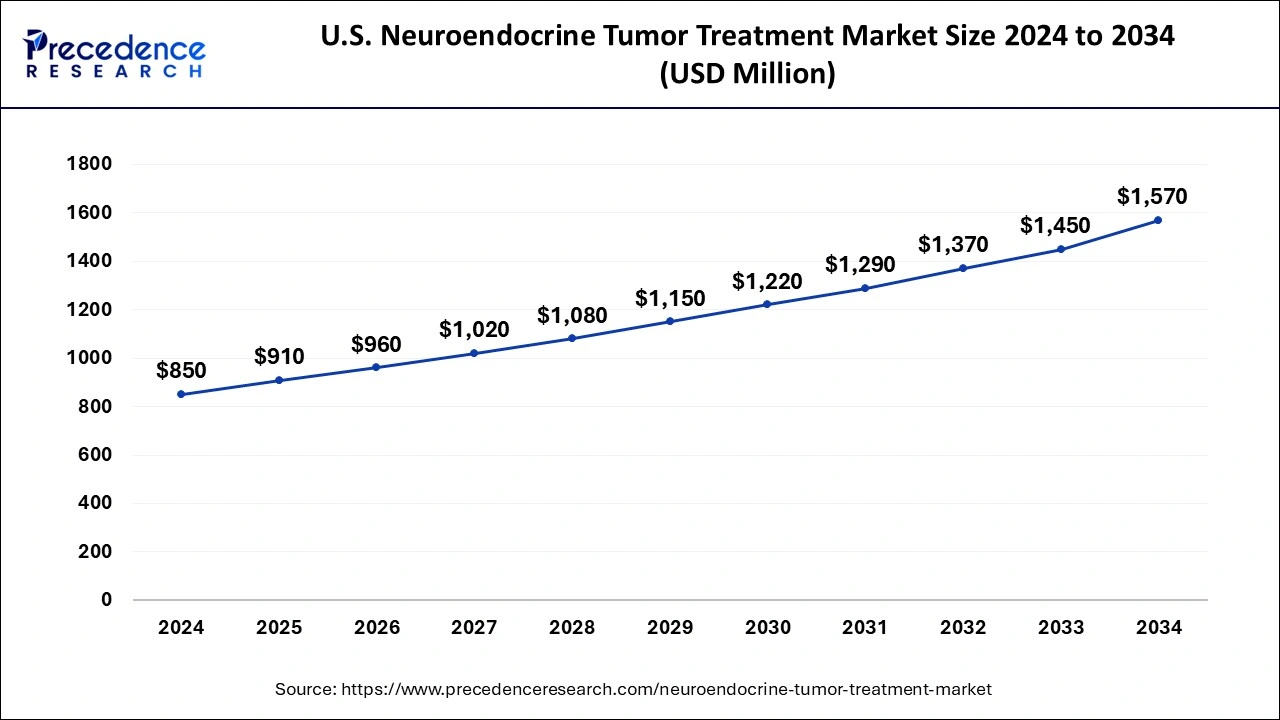
The U.S. neuroendocrine tumor treatment market size is exhibited at USD 910 million in 2025 and is projected to be worth around USD 1,570 million by 2034, growing at a CAGR of 6.32% from 2025 to 2034.
North America dominated the global neuroendocrine tumor treatment market with the largest market share in 2024 for several reasons, including an expanded, well-established healthcare sector, pharmaceutical companies, government investments in research and development firms, and strict regulations. The government of North America has diverted its focus toward research and development of cutting-edge techniques for early detection and diagnostics in oncology due to the rapidly increasing prevalence of neuroendocrine tumor cases in the region.
The advanced healthcare infrastructure and providing access to advanced diagnostic and therapeutic technologies. The United States is leading the regional market due to a stringent regulatory framework that invests in the development of innovative neuroendocrine tumor treatments.
- In April 2024, lutetium Lu 177 dotatate got the Food and Drug Administration (FDA) approval for 12 years and older pediatric patients with somatostatin receptor (SSTR)-positive gastroenteropancreatic neuroendocrine tumors, including foregut, midgut, and hindgut neuroendocrine tumors.
Europe is anticipated to witness significant growth in the neuroendocrine tumor treatment market during the forecast period due to the vast pharmaceutical infrastructure in the region. Government support and funding for research and developments in the pharmaceutical and healthcare sectors are the key factors enhancing market growth in the region. The prevalence of neuroendocrine tumor treatments has rapidly increased in Europe. The presence of well-established healthcare systems in Europe is allowing access to advanced therapies. Additionally, regions are drifting in market success due to the availability of high-quality healthcare professionals.
Regulatory initiatives like the European Medicines Agency (EMA) are supporting the development of novel, innovative treatment options. Moreover, companies are easily launching their novel treatment innovations with the support of a harmonized regulatory environment. The ongoing collaborations between universities, cancer centers, and research institutes are further highlighting innovations. Additionally, partnerships between institutes and organizations are developing and commercializing new NET treatments.
- In September 2024, Ipsen showcased final data from the CABINET Phase III trial investigating Cabometyx versus placebo in patients with advanced pancreatic neuroendocrine tumors (pNETs) or advanced extra-pancreatic neuroendocrine tumors (epNETs) whose disease has advanced after prior systemic therapy. These results show that Cabometyx minimizes the progressions of disease or mortality by 77% for persons with advanced pNETs and 62% for epNETs.
Asia Pacific Neuroendocrine Tumor Treatment Market: Rapid Growth Driven by Advanced Therapies and Early Diagnosis
The Asia Pacific neuroendocrine tumor (NET) treatment market is experiencing its fastest growth, fueled by rising awareness, increasing incidence rates, and improved diagnostic capabilities. Countries like China, Japan, and South Korea are witnessing rapid adoption of somatostatin analogs and targeted therapies, such as everolimus and sunitinib, in clinical practice. Peptide Receptor Radionuclide Therapy (PRRT) is gaining momentum as hospitals expand access to advanced nuclear medicine treatments.
China Neuroendocrine Tumor Treatment Market Trends
China's market is growing rapidly, driven by rising NET incidence and improved diagnostic capabilities. Somatostatin analogs, along with targeted therapies like everolimus and sunitinib, are becoming more widely used in Chinese clinical practice. Peptide Receptor Radionuclide Therapy (PRRT) is gaining traction in major hospitals as a potent option for advanced, somatostatin-receptor-positive NETs.
What Is Being Propelled into Growth in the Neuroendocrine Tumor Treatment Market in Europe?
Europe is at the forefront of the neuroendocrine cancer treatment space - there are considerable investments in clinical and R&D, good reimbursement policies, and sophisticated healthcare systems. For example, Germany, the UK, France, and Italy have ongoing leading clinical trials and early access protocols for new therapies, including Lutathera and everolimus in NET.
A strong cancer research network (clinical and academic), supportive government processes, and funding all encourages not only better patient outcomes, but supports the development of new treatments. Further, the European market continues to be underpinned by the integration of both precision oncology and nuclear medicine.
Germany Neuroendocrine Tumor Treatment Market Trends
Germany's market is advancing strongly, fueled by rising awareness and improved diagnosis through sophisticated imaging like Ga 68 PET/CT. Peptide Receptor Radionuclide Therapy (PRRT) is becoming a mainstay in German NET care, offering targeted treatment for somatostatin-receptor-positive tumors. There is growing adoption of personalized medicine approaches, with therapies tailored based on molecular profiling and tumor subtype.
How Is the Neuroendocrine Tumor Treatment Market Transforming Across Latin America?
The neuroendocrine tumor treatment market across Latin America is progressing at a modest pace, with a corresponding increase in healthcare infrastructure and national efforts towards improvement in cancer diagnostics. Brazil, Mexico, and Argentina are engaging with drug manufacturers through clinical research partnerships. A proactively affordable cancer therapy approach in the region and newly developed private healthcare initiatives to improve advanced therapy access are both helping increase treatment uptake; although, treatment access and awareness continue to hinder rural regions due to high costs.
In what way does the Value Chain Support Neuroendocrine Tumor Treatment in the Global Market?
The value chain supporting treatment for patients with neuroendocrine tumors (NET) integrates research and clinical practice with development and stakeholder engagement to facilitate the timely delivery and innovation of therapy. The value chain involves multiple stakeholders, including pharmaceutical companies, technology companies (HIT and diagnostic companies), hospitals, and regulatory agencies that partner to improve outcomes and the affordability of care.
- Research and Drug Development: The pharmaceutical industry and biotechnology companies continue to invest heavily to facilitate molecular diagnostics and genomics. These discoveries enable pharmaceutical companies to identify new and targeted proteins able to result in precision treatments, increase the efficacy of the drug, and reduce the side effects.
- Clinical Trials and Regulatory Approvals: There is a rigorous framework for clinical trials, especially under the supervision and study from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to assess safety and efficacy. The collaboration between oncology centers and pharmaceutical companies expedites the process of collecting data and the timeline for approval of next-generation drugs, and learning more about current treatments available for NET.
- Distribution and Access for Patients: Strategic collaboratives between manufacturers, distributors, and accountable healthcare providers facilitate the global impact and accessibility for patients. Additionally, platforms for digital health and eHealth support the ability to monitor a patient long-term and adjust their adherence to their treatment plan.
Neuroendocrine Tumor Treatment Market Companies

- Novartis
- Ipsen Pharma
- Pfizer Inc.
- Chimeric Therapeutics
- Bristol-Myers Squibb Company
- Hutchison MediPharma Limited.
- AVEO Pharmaceuticals, Inc.
- Bayer
- Boehringer Ingelheim International GmbH
- Sumitomo Dainippon Pharma
- Merck & Co.
- Spectrum Pharmaceuticals
- Exelixis, Inc.
Leaders' Announcements
- In September 2024, Jennifer Chan, M.D., M.P.H., study chair for the CABINET trial, clinical director of the Gastrointestinal Cancer Center, and director of the Program in Carcinoid and Neuroendocrine Tumors at Dana-Farber Cancer Institute, gave a statement on Exelixis, Inc.'s final data from CABINET, a phase 3 pivotal trial. “The National Cancer Institute's National Clinical Trials Network conducted the phase 3 CABINET study, which shows real-world clinical practice, which has enrolled a wide and heterogeneous range of patients regardless of primary tumor site, grade, somatostatin receptor expression, and functional status.”
- In October 2023, Elyse Gellerman, CEO of NETRF, talked about Chimeric Therapeutics CDH17 CAR T cell therapy, CHM 2101, in clinical trials, which was developed from Dr. Hua's lab. “NETRF is proud to have supported Dr. Hua's research for leading this important clinical trial.”
Recent Developments
- In November 2024, the U.S. Food and Drug Administration (FDA) notified Exelixis, Inc. that an Oncologic Drugs Advisory Committee (ODAC) meeting in March 2025 will again discuss the supplemental New Drug Application (sNDA) for cabozantinib for the treatment of adults with previously treated advanced pancreatic neuroendocrine tumors (pNET) and advanced extra-pancreatic NET (spent).
- In October 2024, Boehringer Ingelheim partnered with Circle Pharma (Circle) and generated a license agreement with the shared goals. The aim of collaboration is to develop a first-in-class cyclin inhibitor, which will be able to stop the growth of cancer cells.
- In October 2023, the FDA provided approval for Chimeric Therapeutics, an Australian leader in cell therapy, CHM 2101, which was developed in Dr. Hua's lab, for a clinical trial to begin patient admissions in 2024. CHM 2101 is projected to be the first CDH17 CAR T cell therapy in clinical trials, potentially in the treatment of NETs.
Segments Covered in the Report
By Type
- Meningiomas
- Adrenal Cancer
- Carcinoid Tumors
- Paraganglioma
- Pheochromocytoma
- Others
By Product
- Somatostatin Analogs (SSAs)
- Targeted Therapy
- Others
By Site
- Lung
- Pancreas
- Colon
- Small Intestine
- Rectum
- Stomach
- Others
By End-use
- Hospitals
- Clinics
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting