Neurological Rare Disease Biologics Market Size and Forecast 2025 to 2034
The global neurological rare disease biologics marketis witnessing rapid growth as biologic therapies target rare and complex nervous system disorders. These treatments offer new hope for underserved patient groups.The market is growing due to the increasing prevalence of rare neurological disorders and the rising adoption of targeted biologic therapies, which offer improved efficacy and safety compared to conventional treatments.
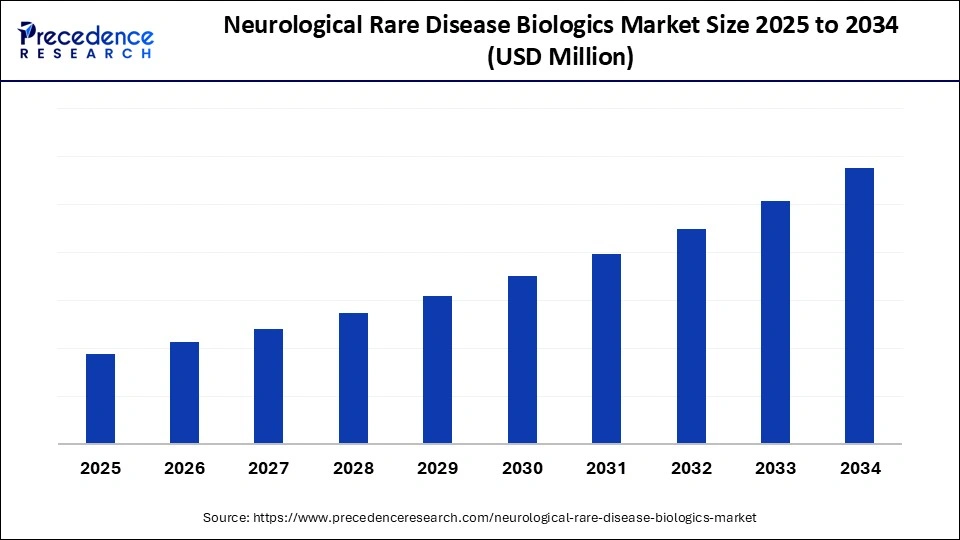
Neurological Rare Disease Biologics Market Key Takeaways
- North America dominated the neurological rare disease biologics market in 2024.
- Asia Pacific is expected to grow at the fastest rate during the forecast period.
- By disease type, the spinal muscular atrophy (SMA) segment held the largest share in 2024.
- By disease type, the Rett syndrome & rett-like disorders segment is expected to grow at the fastest rate during the forecast period.
- By therapy type, the oligonucleotide therapeutics segment led the market in 2024.
- By therapy type, the gene therapies segment is projected to grow at the fastest CAGR during the forecast period.
- By drug class/mechanism, the exon-skipping ASOs segment captured the biggest market share in 2024.
- By drug class/mechanism, the CRISPR/based editing modalities segment is the fastest-growing during the forecast perio.
- By product type, the originator-branded biologics & gene therapies segment held the largest market share in 2024.
- By product type, the platform therapeutics segment is emerging as the fastest-growing during the forecast period.
- By distribution channel, the specialty pharmacies segment generated the major market share in 2024.
- By distribution channel, the hospital segment is expected to grow at the fastest CAGR during the forecast period.
Market Overview
The neurological rare disease biologics market is experiencing strong growth, brought in by the growing use of sophisticated biologic treatments and the occurrence of uncommon neurological conditions. Alternatives to traditional medications that are more focused, efficient, and secure include gene therapies, enzyme replacement therapies, and monoclonal antibodies. The market is expanding more quickly due to favorable regulatory frameworks, rising R&D investments, and increased patient and physician awareness. Additionally, the market is positioned for sustained growth as a result of expanded access to these expensive biologic therapies worldwide, made possible by better healthcare infrastructure and reimbursement support.
How Is AI Impacting Target Discovery for the Neurological Rare Disease Biologics Market?
Artificial intelligence is revolutionizing the hunt for valid biological targets in the neurological rare disease biologics market by integrating multi-omics data, patient Phen clustering, and predictive modeling to accelerate the identification and validation of high-potential therapeutic candidates. Instead of taking years to produce actionable insights, these AI systems can sort through intricate genetic transcriptomic and clinical datasets to identify hidden disease drivers and categorize patient subgroups. Crucially, AI makes it possible for closed-loop validation workflows that pair in silico predictions with functional screens like CRISPR to verify that targets not only seem realistic but also produce significant biological effects in pertinent models.
- In November 2024, Citizen Health announced that it had received more seed funding and a partnership aimed at combating rare diseases. Artificial-intelligence-powered health platforms are aiming to be a liaison between patient communities, which can provide data for preclinical development and pharmaceuticals. (Source: https://www.fiercebiotech.com)
Neurological Rare Disease Biologics MarketGrowth Factors
- Rising Prevalence of Rare Neurological Disorders: More patients are being diagnosed, increasing demand for specialized biologic treatments.
- Advancements in Biologic Therapies: Innovative therapies like monoclonal antibodies and gene therapies offer better efficacy and safety than conventional drugs.
- Favorable Regulatory Support: Orphan drug incentives and expedited approvals encourage companies to invest in rare disease biology.
- Increasing R&D Investment: Collaborations and funding are accelerating the development of new therapies.
- Growing Awareness: Physicians and patients are more informed about rare neurological diseases and are aware of available biologic treatments.
- Rising Healthcare Spending: Improved infrastructure and reimbursement policies support the adoption of costly biologics.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Disease Type, Therapy Type, Drug Class / Mechanism, Product Type, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Advances in Genetic Research and Precision Medicine
Biologics created especially for uncommon neurological disorders are now possible thanks to ongoing advancements in precision medicine and genetic sequencing. The development of treatments like gene therapy, antisense oligonucleotides, and monoclonal antibodies that target the underlying cause rather than the symptoms is aided by the early detection of disease-causing mutations. The shift is enabling personalized treatment strategies that were previously impossible and is increasing success rates in clinical trials for orphan neurologic diseases. The availability of precision-based platforms also lowers the risk of trial failure and boosts investor confidence in the neurological rare disease biologics market.
- In June 2025, SineuGene Therapeutics announced that its gene therapy candidate SNUG01 received FDA Orphan Drug Designation for ALS.(Source: https://www.biospace.com)
Improved Diagnosis and Early Detection
Rare neurological disorders are being detected earlier thanks to increased newborn screening, improved diagnostic equipment, and awareness campaigns. The patient pool becomes more defined as diagnosis rates rise, increasing the viability and accessibility of biologic treatment. Early detection not only improves treatment outcomes but also justifies investment in high-cost biologics. This growing visibility of patient populations encourages payers and regulators to support innovative therapies.
- In December 2023, Nevada began universal newborn screening for SMA, becoming the 49th state to do so.(Source: https://www.curesma.org)
Restraints
High Development and Manufacturing Costs
Creating biologics for uncommon neurological conditions requires sophisticated research platforms, intricate biologics manufacturing processes, and specialized knowledge because of their small population and lengthy development period. Research and development have a significantly higher per-patient cost compared to other common diseases. As a result, smaller businesses are unable to compete, and larger businesses' budgets are severely strained. Cost containment is a persistent problem because biologics manufacturing facilities for viral vector and cell therapies are limited and costly to run.
Complex Regulatory and Ethical Barriers
Incentives notwithstanding, regulatory bodies require thorough safety and effectiveness testing, particularly for treatments that target the central nervous system. Due to small trial sizes, rare-disease biologics frequently struggle to show statistically significant results. The use of placebos in serious pediatric conditions is one example of an ethical dilemma that makes approval even more difficult. Even for treatments with encouraging early data, these obstacles may result in longer timeframes and higher costs overall, leading to the lagging behind of the neurological rare disease biologics market.
Opportunities
Growing Focus on Orphan Drug Designations and Incentives Biologics Investment
The development for rare neurological diseases is being promoted by governments and regulatory agencies worldwide through the provision of tax credits, fee waivers, market exclusivity, and expedited approvals. Biopharma companies benefit from these incentives by having lower financial risks and faster time-to-market for new treatments. Biologic developers now have excellent commercial prospects as the orphan drug ecosystem continues to grow, driven by increasing advocacy campaigns and patient organizations advocating for access.
Advancements in Gene and Cell Therapy Platforms
The adoption of advanced biologic modalities such as AAV-based gene therapies, antisense oligonucleotides, and CAR-T-like platforms for neurology is creating breakthrough opportunities. These technologies can address the underlying genetic causes of rare diseases, offering one-time curative potential instead of chronic symptom management. As manufacturing scales and delivery methods improve, the time spent in the pipeline for the neurological rare disease biologics market products is reducing drastically.
Disease Type Insights
Why Did the Spinal Muscular Atrophy (SMA) Segment Dominate the Neurological Rare Disease Biologics Market in 2024?
The spinal muscular atrophy (SMA) segment dominated the neurological rare disease biologics market in 2024, primarily because of the accessibility of well-known and authorized biologics like Evrysdi, Spinraza, and Zolgensma. Because SMA can be fatal in infants and children, it has garnered significant clinical attention and funding, which has accelerated the adoption of cutting-edge treatments. Early diagnosis and prompt treatment initiation were made possible by the existence of strong newborn screening programs in multiple nations. Long-term research partnerships and helpful payment plans also improved patient access to treatments. All these elements worked together to guarantee SMA's dominance in this market.
Rett syndrome & rett-like disorders emerged as the fastest growing segment in 2024 as new biologics and gene therapies were developed more quickly as a result of research breakthroughs. Investment opportunities were facilitated by the dearth of treatment alternatives and the substantial unmet medical needs associated with these conditions. Innovative gene editing and RNA-based platforms are being prioritized by businesses more and more in an effort to address the underlying genetic mutations. Additionally important in raising awareness and promoting clinical trial participation have been patient advocacy groups. Rett-related biologics are anticipated to experience rapid growth in the upcoming years due to increasing regulatory support for orphan indications.
Therapy Type Insights
Why Did Oligonucleotide Therapeutics Lead the Neurological Rare Disease Biologics Market in 2024?
Oligonucleotide therapeutics dominated the market in 2024, inspired by their demonstrated clinical effectiveness in diseases such as DMD and SMA. Their ability to scale and adapt to target various genetic mutations has guaranteed their market leadership and broad adoptionSuccessful commercial launches and regulatory approvals have further reinforced their competitive advantages. Their dominance will probably be solidified if research pipelines are expanded to include more indications.
Gene therapies are the fastest-growing segment, motivated by the possibility of providing one-time permanent treatments increase in investments and regulatory approvals is expediting the commercialization of viral vector technologies. They are becoming even more relevant in rare neurological diseases as precision medicine gains more attention. Gene therapies are predicted to revolutionize the treatment paradigm as a result of growing trial successes.
Drug Class/Mechanism Insights
Why Did the Exon Skipping ASOs Segment Dominate the Market in 2024?
The exon-skipping ASOs dominated the neurological rare disease biologics market because of their proven ability to treat neuromuscular conditions like DMD. Their growing late-stage pipeline and clinical success have guaranteed widespread physician and patient adoption. Regulatory frameworks that are supportive of orphan drugs have also increased their use. Mutation-specific products are expected to continue to dominate the market as more of them are approved.
CRISPR/Based editing modalities are the fastest-growing class as they directly target and repair faulty genes. Advancements in delivery platforms and strong investor interest are rapidly moving them closer to commercial use. Their potential to offer curative outcomes has positioned them at the forefront of innovation. Ongoing global collaborations are further speeding up their clinical progress.
Product Type Insights
Why Did the Originator-Branded Biologics & Gene Therapies Segment Lead the Market in 2024?
Originator-branded biologics & gene therapies dominated in 2024 due to their proven efficacy, safety, and reimbursement systems. Supported by robust intellectual property and physician confidence, they maintain their dominant market share. Patient confidence has increased as a result of significant marketing campaigns by top pharmaceutical companies. They will always be preferred over alternatives due to their solid clinical record.
The platform therapeutics segment is the fastest-growing product type, as it enables scalable drug development across multiple rare diseases. Their cost efficiency and ability to streamline pipelines make them highly attractive for companies and investors alike. Increased adoption of RNA-based and viral-vector platforms is driving momentum. These platforms are expected to unlock opportunities for treating ultra-rare conditions.
Distribution Channel Insights
Why Did the Specialty Pharmacies Segment Dominate the Neurological Rare Disease Biologics Market in 2024?
The specialty pharmacies dominated the neurological rare disease biologics market in 2024 due to their proficiency in handling complicated biologics for uncommon neurological conditions. Their role has been reinforced by their capacity to offer cold-chain logistics adherence monitoring and patient education. Drug manufacturer partnerships improve patients' access to cutting-edge treatments even more. They are essential to the distribution of biologics because of their specialized services.
The hospital segment is a growing channel as advanced biologics and gene therapies often require inpatient or specialized administration. Their infrastructure supports infusion services, genetic testing, and multidisciplinary care for rare diseases. Growing hospital-based clinical trials are further increasing therapy availability. Hospitals are becoming central hubs for managing next-generation biologics.
Regional Insights
North America dominated the neurological rare disease biologics market in 2024, driven by early adoption of novel treatments and a sophisticated healthcare infrastructure. Patients now have better access to treatments thanks to robust orphan drug policies and reimbursement schemes. The development and commercialization of therapies have accelerated due to the presence of global biotech leaders. The region's ascendancy is further reinforced by growing patient advocacy and awareness initiatives. Innovation in rare disease research and clinical trials is being further stimulated by increased government funding. North America is anticipated to maintain its market leadership over time due to its strong ecosystem.
Asia Pacific is the fastest-growing region, driven by growing awareness of rare diseases and increased investments in healthcare infrastructure. For orphan medications, governments are implementing expedited procedures and policies that are supportive. Growing partnerships with pharmaceutical firms are improving the accessibility of biologics. It is expanding quickly due to a sizable patient base and enhanced diagnostic capabilities. Regulatory approvals and trial activity are being expedited by the regions' expanding clinical research centers. The Asia Pacific region is emerging as a major growth frontier for market participants due to the growing adoption of advanced biologics.
Neurological Rare Disease Biologics Market Companies

- Alexion (AstraZeneca)
- Amgen
- Biogen
- Bluebird bio
- Catalent
- Eli Lilly
- Ionis Pharmaceuticals
- Novartis
- Pfizer
- Regenxbio
- Roche / Genentech
- Sanofi
- Sarepta Therapeutics
- Stoke Therapeutics
- UCB
Recent Developments
- In September 2025, the U.S. FDA announced a new proposed pathway, the Rare Disease Evidence Principles (RDEP), to expedite the approval of therapies for ultra-rare genetic disorders. This process allows the use of a single, adequate trial, such as a single-arm study supported by confirmatory that, significantly easing regulatory requirements for rare-disease biologics.(Source: https://www.reuters.com)
- In February 2025, IXICO plc announced further validation of its AI-driven 'IXIQ.Ai' imaging platform for Huntington's Disease. Developed under the Huntington's Disease Imaging Harmonisation consortium, the platform enhances biomarker detection and offers new avenues for tracking disease progression and therapeutic impact.(Source: https://ixico.com)
Segments Covered in the Report
By Disease Type
- Spinal Muscular Atrophy (SMA)
- Hereditary Neuropathies (e.g., Charcot-Marie-Tooth)
- Lysosomal Storage Disorders with Neurological Involvement
- Rett Syndrome & Rett-like Disorders
- Hereditary Ataxias (e.g., Friedreich's, Spinocerebellar)
- Rare ALS & Motor Neuron Diseases
- Huntington's Disease Subpopulations
- Ultra-Rare Pediatric Neurodegenerative Disorders
By Therapy Type
- Gene Therapies (AAV, Lentiviral, Gene Editing)
- Oligonucleotide Therapeutics (ASOs, siRNA, Splice Modulators)
- Monoclonal Antibodies & Biologic Proteins
- Enzyme Replacement Therapies (ERT)
- Cell Therapies (Neural Stem Cells, MSCs, CAR-T Variants)
- Therapeutic Proteins & Peptides
By Drug Class / Mechanism
- Exon-Skipping ASOs
- RNAi Therapeutics (siRNA)
- AAV-Based Gene Replacement
- CRISPR/Base Editing Modalities
- Recombinant Enzymes & Fusion Proteins
- Neurotrophic Factor Agonists
- Anti-Neuroinflammatory mAbs
By Product Type
- Originator Branded Biologics & Gene Therapies
- Biosimilars & Follow-On Biologics
- Platform Therapeutics (Licensed Programs)
- Cell Therapy Products
By Distribution Channel
- Hospital Procurement
- Specialty Pharmacies
- Online Pharmacies
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content