What is the Preeclampsia Diagnostics Market Size?
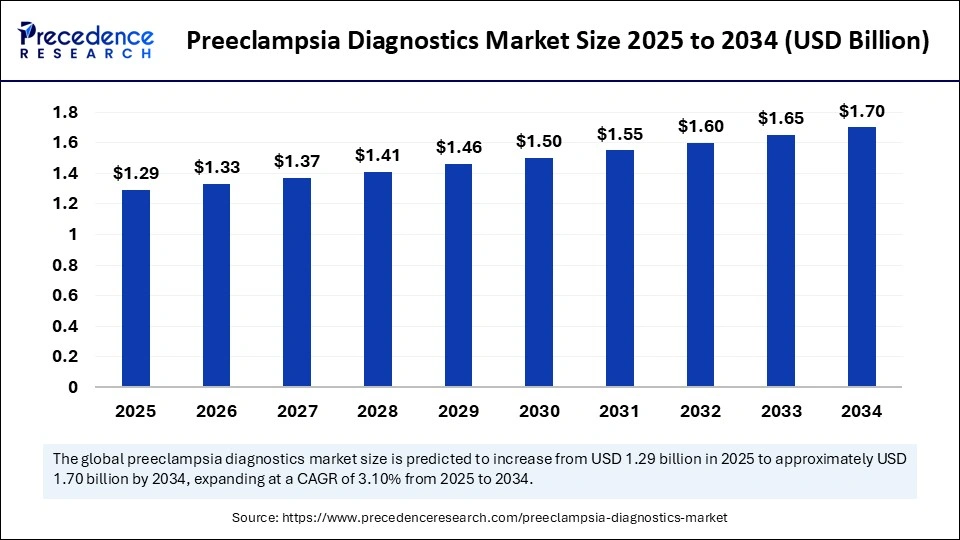
The global preeclampsia diagnostics market size accounted for USD 1.29 billion in 2025 and is predicted to increase from USD 1.33 billion in 2026 to approximately USD 1.70 billion by 2034, expanding at a CAGR of 3.10% from 2025 to 2034. The market is expanding due to rising awareness of maternal health, technological advancements in point-of-care testing, and increasing prenatal screening initiatives.
Market Highlights
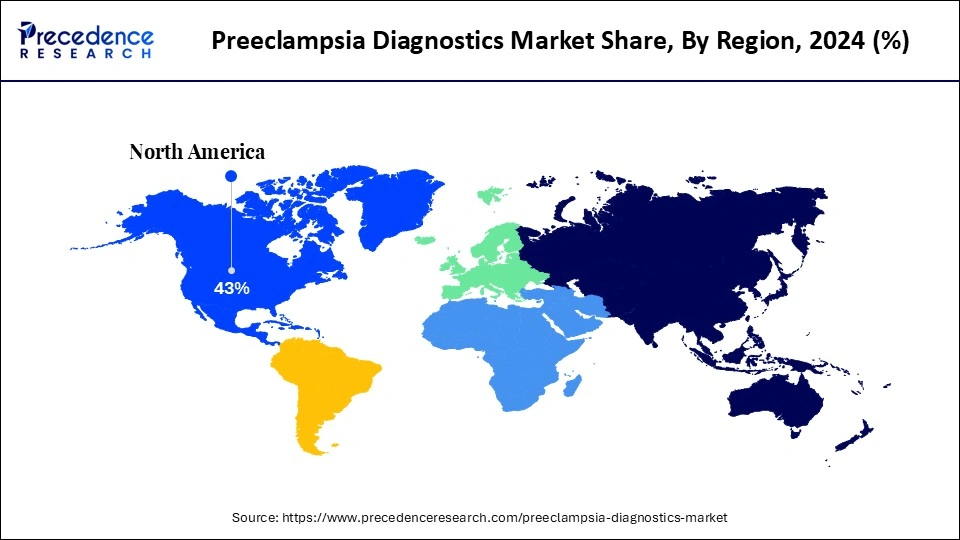
- North America led the preeclampsia diagnostics market with around 43% share in 2024.
- Asia Pacific is expected to expand at the fastest CAGR in the market between 2025 and 2034.
- By test type, the biochemical marker assays segment held approximately 40% share of the market in 2024.
- By test type, the point-of-care testing segment is expected to grow at the fastest CAGR between 2025 and 2034.
- By technology type, the immunoassays segment captured around 45% market share in 2024.
- By technology type, the biosensors / point-of-care devices segment is expected to expand at a notable CAGR over the projected period.
- By test setting, the laboratory-based testing segment captured approximately 50% market share in 2024.
- By test setting, the home-based / remote monitoring segment is expected to expand at a notable CAGR over the projected period.
- By product & services, the diagnostic kits & reagents segment held around 45% of market share in 2024.
- By product & services, the data analytics & monitoring platforms segment is expected to expand at a notable CAGR over the projected period.
- By end user, the hospitals & maternity clinics segment held approximately 50% of the market share in 2024.
- By end user, the obstetrics & gynecology clinics segment is anticipated to grow at a fastest CAGR between 2025 and 2034.
Market Size and Forecast
- Market Size in 2025: USD 1.29 Billion
- Market Size in 2026: USD 1.33 Billion
- Forecasted Market Size by 2034: USD 1.70 Billion
- CAGR (2025-2034): 3.10%
- Largest Market in 2024: North America
- Fastest Growing Market: Asia Pacific
Strategic Overview of the Global Preeclampsia Diagnostics Industry
The preeclampsia diagnostic market is consistently expanding, as the rising number of hypertensive disorders of pregnancy and increasing awareness of early identification of maternal complications contribute to overall acceptance. The preeclampsia diagnostics market includes diagnostic tools, assays, devices, and laboratory tests used to detect and monitor preeclampsia, a pregnancy-related hypertensive disorder with serious maternal and fetal complications. The market encompasses blood pressure monitoring, biochemical markers, urine protein tests, imaging-based diagnostics, and point-of-care testing. Innovations such as predictive algorithms, point-of-care devices, and home-based monitoring platforms are further expanding adoption, improving clinical outcomes, and reducing maternal and neonatal morbidity globally.
Artificial Intelligence: The Next Growth Catalyst in Preeclampsia Diagnostics
Artificial Intelligence (AI) is increasingly being applied in the preeclampsia diagnostics market, with predictive risk assessment emerging as a major area of focus. AI algorithms are being developed to analyze complex patient data, including blood biomarkers, maternal medical history, and placental imaging, to identify pregnancies at high risk for preeclampsia well before clinical symptoms manifest. For example, a 2024 study by the University of Oxford and King's College London demonstrated that an AI-based model could predict preeclampsia with high accuracy, showcasing its potential for early intervention.(Source: https://www.kcl.ac.uk)
Furthermore, nationally funded initiatives like the NIH's Human Placenta Project are exploring how machine learning can process molecular and imaging data to enable non-invasive diagnostic approaches. The broader integration of AI into prenatal care not only holds promise for maximizing early detection but also supports individualized monitoring and timely interventions, ultimately improving maternal and fetal health outcomes. This positions AI as a transformative tool in advancing the effectiveness and accessibility of preeclampsia diagnostics.
Preeclampsia Diagnostics Market Outlook
- Market Growth Overview: The market is set for rapid growth between 2025 and 2034 due to the rising prevalence of preeclampsia. The WHO estimates that preeclampsia affects approximately 2-8% of pregnancies globally and is responsible for an estimated 46,000 maternal deaths annually, creating a strong market for early diagnostic solutions to identify preeclampsia and provide appropriate care. The rising integration of technologies to improve diagnostic accuracy and accessibility through biomarkers and AI-based screening platforms further support market growth.
- Research & Development: Ongoing research is heavily focused on biomarker-based detection, particularly the sFlt-1/PlGF ratio, and molecular-level studies for improved risk stratification. This shift is supported by major funding bodies like the NIH and EU maternal health initiatives, enabling a transition from reactive diagnostics to predictive and preventative models aimed at reducing maternal and neonatal morbidity and mortality.
- Industry Leaders: Key diagnostic companies are actively engaging with regulatory agencies to fast-track the clinical adoption of validated biomarkers, fostering greater trust in the medical community. Collaborative efforts between diagnostic developers, academic institutions, and healthcare organizations are accelerating product validation and commercialization, particularly for both developed and emerging healthcare markets.
- Government and Policy Development: Global health organizations such as the WHO and CDC are advocating for standardized screening protocols and equitable access to preeclampsia diagnostics. Policy frameworks that incentivize R&D funding for maternal health and promote public-private partnerships are expected to drive procurement and implementation of testing solutions, especially in low- and middle-income countries.
- Sustainability Trends: Sustainability trends involve worldwide health equity and accessibility, point-of-care testing, biomarker innovation for invasiveness, and telemedicine and remote monitoring.
- Major Investors: Major investors in the market include The Vanguard Group, Inc., State Street Corporation, FMR LLC, Venture Capital and Investment Firms, Strategic Corporation, and Preeclampsia Foundation.
- Startup Economy: The startup economy is focused on novel biomarkers and genetic testing, AI and machine learning for risk stratification, and home healthcare and tele-prenatal monitoring.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 1.29 Billion |
| Market Size in 2026 | USD 1.33 Billion |
| Market Size by 2034 | USD 1.70 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 3.10% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Technology, Delivery System, Route of Administration, Target Disease, Target Tissue, End-User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising Maternal Mortality Rates
The increase in global maternal mortality burden due to hypertensive disorders in pregnancy is emerging as a key driver of the preeclampsia diagnostics market. According to the WHO, preeclampsia affects 2%-8% of pregnancies, leading to approximately 46,000 maternal deaths and close to 500,000 fetal or newborn deaths each year. In Asia, Africa, and Latin America, preeclampsia accounts for as many as one-quarter of maternal deaths.(Source: https://www.who.int)
As a result, the global public health community, including policymakers, government agencies, and healthcare organizations, has begun prioritizing early screening and diagnostic advancements. The global growth in biomarker-based diagnostic tests, in addition to portable diagnostic kits and devices, is positively influencing early detection and pregnancy outcomes.
Restraint
Limited Biomarker Validation
A key inhibiting challenge for the preeclampsia diagnostics market is that the development of new biomarkers for early detection has received limited clinical validation. For example, sFlt-1/PlGF ratios and RNA-based signatures have demonstrated promising potential for early detection of risk; however, none have undergone extensive clinical trials across multiple ethnic groups. A 2024 portfolio study published on PubMed notes the regulatory challenges of inconsistent validation results when tested against various populations.
- For instance, the Mirvie RNA test was first trialed to predict preterm preeclampsia with an accuracy rating of about 91% based on studies; however, it still awaits eventual validation beyond the original studies across a global community. The absence of standardization and population-wide datasets further contributes to a lack of confidence.(Source: https://www.mirvie.com)
Opportunity
Development of Point-of-Care Diagnostic Kits
Point-of-care (POC) diagnostic kits are changing the way preeclampsia is identified by providing rapid, affordable, and accessible diagnostic testing. These innovations can have a significant impact in low-resource settings, where traditional laboratory-based diagnostics are often lacking.
- For example, in March 2024, the Lumella test utilizes the GlyFN biomarker to enable an easy-to-use clinical test that produces results in just 10 minutes with a single drop of blood, reporting a specificity of 92.8% and a sensitivity of 98.5%. (Source: https://www.aghealth.co.uk)
- The Lepzi PLGF test is another cost-effective solution for detecting preeclampsia-related conditions, as it measures the PlGF biomarker directly from whole-blood samples, thereby contributing to diagnosis and prognosis.(Source: https://unahealth.co.uk)
These types of solutions not only improve early detection but also assist healthcare practitioners in making timely decisions, both of which can lower maternal and neonatal morbidity and mortality in cases of preeclampsia.
Segment Insights
Test Type Insights
Which Test Type Hold the Largest Share of the Market for Preeclampsia Diagnostics?
The biochemical marker assays segment held around 40% share of the preeclampsia diagnostics market in 2024, as hospitals and laboratories heavily utilize biochemical marker tests due to their high accuracy in identifying preeclampsia in the early stages of disease. Biochemical markers have withstood the test of time and have been extensively clinically validated for use in prenatal screening programs globally.
The point-of-care testing segment is expected to grow at the fastest rate in the coming years due to the demand for quicker diagnostic results at the location of testing. Point-of-care tests can identify preeclampsia earlier, in outpatient and remote settings, thereby improving maternal and fetal outcomes. Point-of-care tests with integrated mobile health functionalities, such as simplified blood sample sonication, are expected to continue growing in hospitals and also expand significantly within households and clinics.
Technology Type Insights
Why Did the Immunoassays Segment Lead the Market in 2024?
The immunoassays segment led the preeclampsia diagnostics market, accounting for almost 45% share in 2024. This is primarily due to their widespread adoption, driven by their high sensitivity and specificity in detecting key preeclampsia biomarkers, such as placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1). They are relied upon in hospitals and laboratories to provide evidence-based, reliable, and reproducible results that inform early risk stratification and guide management in high-risk pregnancies.
The biosensors / point-of-care devices segment is likely to expand at the fastest CAGR in the upcoming period. This is primarily due to their portability, real-time monitoring capability, and integration capability with mobile health applications allow for fast detection at outpatient clinics and remote sensing locations. These devices enable convenience for patients, allow for delayed detection, and can continuously monitor patients. They have become of heightened utility in decentralized maternal care.
Test Setting Insights
What Made Laboratory-Based Testing the Dominant Segment in the Market?
The laboratory-based testing segment dominated the preeclampsia diagnostics market, holding about 50% share in 2024. These facilities have high accuracy and validated analysis of any given biomarker; this is why hospitals and specialty diagnostic facilities often prefer to use these laboratories, especially when it comes to a laboratory-based test versus a home-based or monitoring test. Laboratory-based testing also has a controlled and reproducible results environment, which is important with respect to the early detection of preeclampsia or risk-stratifying any given high-risk pregnancy.
The home-based / remote monitoring segment is expected to grow at the fastest rate over the projection period. The increased use of telemedicine, wearable devices, and connected health platforms enables patients to monitor their blood pressure and other vital indicators from the comfort of their own homes. This both increases convenience and reduces visits to medical facilities, promoting timely intervention, especially in remote areas or those with limited resources.
Product & Services Insights
Which Product Segment is Leading the Market?
The diagnostic kits & reagents segment led the preeclampsia diagnostics market with approximately 45% share in 2024. These products help identify biomarkers with precision in both laboratory and hospital settings. These kits are easy to use, reliable, and compatible with standard laboratory equipment, making them a benchmark for prenatal testing, creating consistency and providing early diagnostics in high-risk pregnancies.
The data analytics & monitoring platforms segment is expected to grow at the fastest rate in the coming years. This is due to their ability to transform raw clinical and biological data into actionable insights, enabling early risk prediction and continuous maternal health monitoring. As preeclampsia is a dynamic condition that can rapidly progress, real-time tracking of parameters like blood pressure, proteinuria levels, and biomarker ratios (e.g., sFlt-1/PlGF) becomes critical.
End-User Insights
Which End-User Holds the Largest Share of the Market in 2024?
The hospitals & maternity clinics segment held around 50% share of the preeclampsia diagnostics market in 2024. These end users generally manage a large volume of patients for prenatal care and provide full maternal services, best positioned to take advantage of sophisticated diagnostic testing. Consequently, these facilities have laboratory capabilities and trained staff, including physician assistants/nurse practitioners, as well as an established system for screening their patients in a timely and accurate manner. Comprehensive maternal diagnostics lessen maternal morbidity and fetal complications and enable evidence-based decisions.
The obstetrics & gynecology segment is likely to grow at the fastest rate over the projection period due to its central role in maternal healthcare and prenatal screening. As preeclampsia remains one of the leading causes of maternal and fetal morbidity, obstetricians and gynecologists are often the first point of contact for pregnant women and are thus directly responsible for initiating early diagnostic protocols.
This segment's growth is further driven by the integration of advanced diagnostic tools, including biomarker testing (e.g., sFlt-1/PlGF ratio), placental imaging, and AI-based risk stratification platforms, into routine obstetric care.
Regional Insights
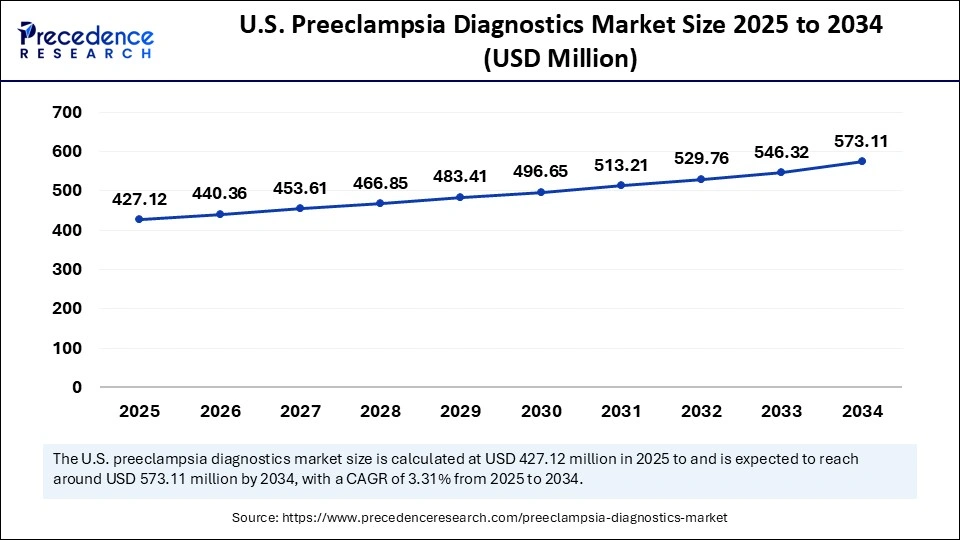
U.S. Preeclampsia Diagnostics Market Size and Growth 2025 to 2034
The U.S. preeclampsia diagnostics market size is evaluated at USD 427.12 million in 2025 and is projected to be worth around USD 573.11 million by 2034, growing at a CAGR of 3.31% from 2025 to 2034.
Why Did North America Lead the Preeclampsia Diagnostics Market in 2024?
North America led the preeclampsia diagnostics market by capturing a 43% share in 2024, driven by its advanced healthcare infrastructure, heightened awareness, and substantial research and development funding. In the United States, robust public-private partnerships and a proactive regulatory environment are fostering diagnostic innovation. For instance, in February 2024, the Foundation for the National Institutes of Health (FNIH) launched a public-private initiative to develop tools that can identify women at high risk for early-onset preeclampsia.
U.S. Preeclampsia Diagnostics Market Trends
The U.S. maintains its leadership through significant investments in maternal health research, a strong focus on personalized medicine, and the early adoption of cutting-edge diagnostics. A key milestone is the January 2024 launch of the PERA test by Mayo Clinic Laboratories, offering 94% sensitivity in detecting preeclampsia. Backed by regulatory initiatives that support rapid diagnostic innovation, these factors collectively reinforce the United States' dominant role in shaping the regional and global market landscape.(Source: https://news.mayocliniclabs.com)
Why is Asia Pacific Considered the Fastest-Growing Region in the Preeclampsia Diagnostics Market?
Asia Pacific is emerging as the fastest-growing market for preeclampsia diagnostics, driven by improved access to healthcare services, rising awareness of maternal health, and increasing investment in diagnostic infrastructure. Countries such as India and China are witnessing significant upgrades in healthcare systems, alongside the adoption of advanced diagnostic technologies. Factors like rapid urbanization, a growing middle-class population, and expansion of maternal care services are further fueling demand for timely and efficient preeclampsia diagnostics.
India Preeclampsia Diagnostics Market Trends
In India, specifically, the market is expanding rapidly due to a large underserved pregnant population and increasing emphasis on early detection and monitoring of high-risk pregnancies. A notable development is the launch of Amazon India's diagnostics service in May 2025, which offers at-home testing solutions with fast sample collection and digital reporting. These innovations are making diagnostics more accessible and timely, particularly in rural and semi-urban regions, thereby contributing to improved maternal outcomes and further market growth. (Source: https://www.aboutamazon.in)
Value Chain Analysis of the Preeclampsia Diagnostics Market
- Research and Development (R&D): This phase involves the identification of biomarkers, the development of diagnostic kits, and the validation of diagnostic test accuracy through clinical trials and partnerships among biotechnological innovation and clinical healthcare institutions.
- Raw Material and Component Sourcing: Suppliers coordinate the provision of essential materials, including but not limited to reagents, antibodies, biosensors, and diagnostic kits. Intermediaries require vendors to provide assurance of high purity and acknowledged physician-grade quality for reliability and long-term diagnostic performance.
- Manufacturing and Production: Diagnostics device manufacturers or laboratories generally produce test kits, point-of-care systems, and consumables in accordance with stringent regulatory guidelines to enable rationalized practices throughout the supply chain, ensuring mass-consistent reliability, sterility of performance, and size consistency across batches.
- Distribution of Platforms in Healthcare Settings: Comprehensive third-party manufacturers have multiple distributors, wholesalers, providers, and healthcare supply chains to expedite the delivery of diagnostic product availability to hospitals, clinics, and labs to stay current on treatment intervention options; however, the delivery almost always requires a cold-chain process for the maintenance of the living biosensor or components like reagents.
- End User/Service Delivery: Hospitals, diagnostic centers, and maternity-level clinics utilize these tests or living notebooks for early detection and monitoring of pre-eclampsia (e.g., with pregnancy screening for pre-eclampsia) to determine timely treatment approaches and improve maternal-fetal outcomes.
Key Players Operating in the Preeclampsia Diagnostics Market and Their Offering
| Tier | Companies in Tier | Approx % Cumulative Share | Commentary |
| Tier I - Market Leaders (~45 55%) | Thermo Fisher Scientific; F. Hoffmann La Roche Ltd.; PerkinElmer Inc. | ~?50% | These firms dominate via strong biomarker assay platforms (especially sFlt 1/PlGF), large global lab networks, regulatory approvals, and broad product portfolios. |
| Tier II - Established Players (~20 30%) | Siemens Healthineers; Abbott Laboratories; Bayer AG; QuidelOrtho Corporation | ~?25% | These contribute significantly via diagnostics instruments, rapid/point of care platforms, complementary assays, and expanding reach into emerging markets. |
| Tier III - Emerging / Niche Players (~15 20%) | DRG Instruments GmbH; Diabetomics, Inc.; Metabolomic Diagnostics Ltd.; Sera Prognostics | ~?15% | These smaller or more specialized companies often focus on risk score tools, early detection/prognostic assays, or regional deployment. |
Recent Developments
- In April 2025, Mirvie announced its new, straightforward RNA-based blood test can identify distinct molecular signatures to predict preeclampsia risk months in advance, with results ranging from high to low. (Source: https://www.preeclampsia.org)
- In January 2024, Labcorp introduced a blood test (B·R·A·H·M·S sFlt-1/PlGF KRYPTOR) that has received FDA clearance to determine whether a pregnant woman is at risk of progressing to severe preeclampsia. (Source: https://ir.labcorp.com)
- On April 30, 2025, IIT-Madras and its collaborators shared a portable fiber-optic biosensor test that can identify a key protein biomarker in just 30 minutes, enabling the detection of early preeclampsia risk during pregnancy.(Source: https://www.business-standard.com)
Expert Analysis
The preeclampsia diagnostics market is poised for robust expansion driven by escalating prevalence of hypertensive disorders during pregnancy and heightened clinical emphasis on early detection to mitigate maternal-fetal morbidity and mortality. The evolving diagnostic landscape, underpinned by advancements in biomarker-based assays, particularly the sFlt-1/PlGF ratio, integrated with innovative point-of-care and home-based testing platforms, is catalyzing market penetration across both developed and emerging geographies. Substantial influxes of public and private funding, coupled with regulatory incentives and collaborative frameworks among biotech firms, research institutions, and healthcare providers, are accelerating translational research and commercial scalability of predictive diagnostics.
Emerging opportunities reside in the confluence of AI-driven predictive analytics, multi-omics biomarker discovery, and digital health integration, enabling personalized risk stratification and real-time monitoring. Moreover, expanding healthcare infrastructure and increasing maternal health awareness in Asia Pacific and Latin America signal high-growth trajectories, supported by governmental policy reforms and investment in maternal care innovation. Despite challenges related to heterogeneity in clinical presentation and variability in biomarker specificity, ongoing R&D initiatives targeting multiplexed assay development and standardized screening protocols are expected to consolidate diagnostic accuracy and market adoption.
In summary, the preeclampsia diagnostics market is entering a transformative phase characterized by technological convergence, regulatory facilitation, and expanding global demand, rendering it an attractive domain for stakeholders aiming to capitalize on unmet clinical needs through innovative, scalable, and accessible diagnostic solutions.
Top Companies in the Preeclampsia Diagnostics Market & Their Offerings:
- Thermo Fisher Scientific: Thermo Fisher provides a wide range of diagnostic products and technologies, including the instruments and consumables used in laboratories to run preeclampsia tests.
- F. Hoffmann-La Roche Ltd. (Roche): Roche contributes significantly to the preeclampsia diagnostics market through its development and commercialization of diagnostic tests, notably those using the sFlt-1/PlGF ratio (soluble fms-like tyrosine kinase-1/placental growth factor) biomarkers.
- PerkinElmer Inc. (now part of Bio-Techne): PerkinElmer has a notable presence through its diagnostic product portfolio, which includes screening and detection kits for various pregnancy-related conditions, including preeclampsia.
- Siemens Healthineers: Siemens Healthineers provides in vitro diagnostic equipment and tests used in clinical laboratories for various conditions, including some general blood pressure monitors and clinical assays related to preeclampsia risk assessment.
- Abbott Laboratories: Abbott offers a wide range of diagnostic solutions and testing equipment used in hospital and clinical settings to monitor the general health parameters relevant to preeclampsia.
- Bayer AG: Bayer contributes through its pharmaceutical and consumer health divisions, offering treatments for conditions that might arise from preeclampsia, such as blood pressure management medications.
- QuidelOrtho Corporation: QuidelOrtho is a major diagnostics company that provides a wide range of in vitro diagnostic solutions and clinical laboratory products used in hospitals and clinics.
- DRG Instruments GmbH: DRG specializes in diagnostic assays, including specific ELISA kits and other tests for biomarkers like PlGF and sFlt-1, which are central to preeclampsia diagnosis.
- Diabetomics, Inc. (now Miraca Holdings): This company previously focused on developing non-invasive diagnostic tests, including an early screening platform for preeclampsia based on specific biomarkers.
- Metabolomic Diagnostics Ltd. (now part of PerkinElmer): The company developed a preeclampsia screening test based on a specific panel of metabolomic biomarkers identified in early pregnancy blood samples.
- Sera Prognostics: Sera Prognostics is a leader in using predictive biomarker technologies for maternal and premature birth health risks, including tests related to preeclampsia.
Segments Covered in the Report
By Test Type
- Blood Pressure Monitoring
- Biochemical Marker Assays
- Placental Growth Factor (PlGF)
- sFlt-1 / sEng Ratio
- Others
- Urine Protein Tests
- Imaging-Based Diagnostics (Ultrasound / Doppler)
- Point-of-Care Testing
- Others
By Technology Type
- Immunoassays
- ELISA / Chemiluminescence-based
- Molecular / PCR-based Assays
- Biosensors / Point-of-Care Devices
- Ultrasound / Imaging-based
- Others
By Test Setting
- Laboratory-based Testing
- Point-of-Care / Bedside Testing
- Home-based / Remote Monitoring
By Product & Services
- Diagnostic Kits & Reagents
- Testing Devices & Instruments
- Laboratory Services
- Data Analytics & Monitoring Platforms
- Others
By End User (no sub-segments)
- Hospitals & Maternity Clinics
- Diagnostic Laboratories
- Obstetrics & Gynecology Clinics
- Research & Academic Institutes
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting