What is the Scleroderma Therapeutics Market Size?
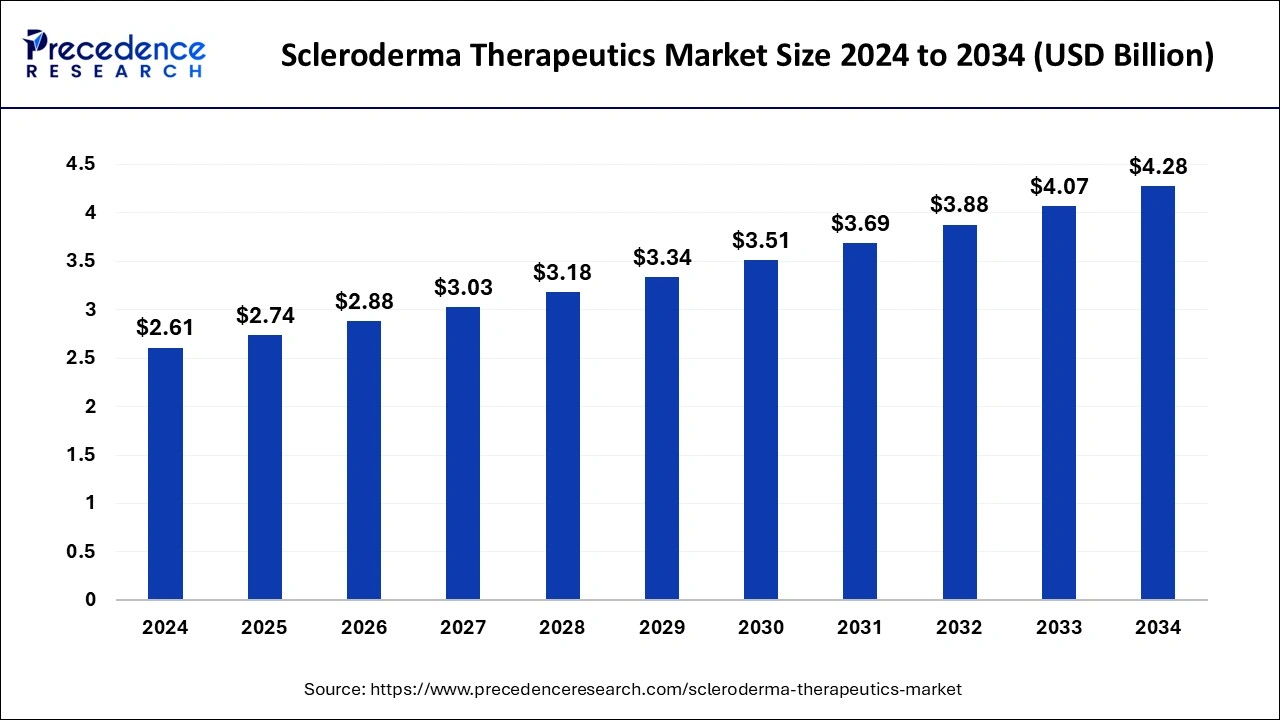
The global scleroderma therapeutics market size is calculated at USD 2.74 billion in 2025 and is predicted to increase from USD 2.88 billion in 2026 to approximately USD 4.48 billion by 2035, expanding at a CAGR of 5.04% from 2026 to 2035.
Scleroderma Therapeutics Market Key Takeaways
- North America dominated the scleroderma therapeutics market with the largest market share of 47% in 2025.
- Asia Pacific is projected to grow at a CAGR of 7.82% during the forecast period.
- By drug class, the immunosuppressors segment contributed the highest share of 29% in 2025.
- By drug class, the endothelin receptor antagonist segment is expected to grow at a CAGR of 5.72% during the forecast period.
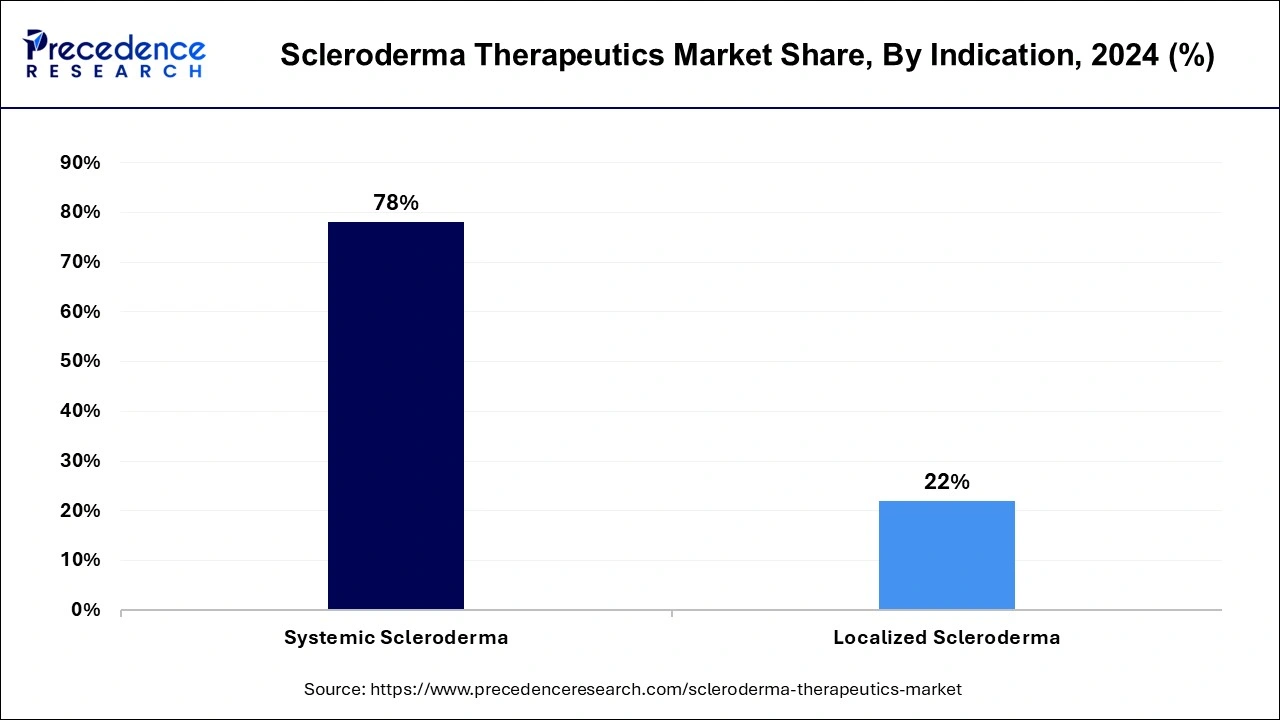
- By indication, the systemic scleroderma segment held a major market share of 78% in 2025.
- By indication, the localized scleroderma segment is anticipated to grow at the highest CAGR during the forecast period.
What are Scleroderma Therapeutics?
Scleroderma is a rare condition in which overproduction of collagen causes a hardening of the skin, and in some cases, blood vessels and internal organs also get affected. The disease is four times more common in women than men. The growing prevalence of scleroderma disease is driving growth in the development of novel treatment solutions. The scleroderma therapeutics market is attributed to North America and Europe due to the increased prevalence of scleroderma in these regions. Moreover, expanded healthcare infrastructure and aging populations are fueling demands for effective treatments for sclerodermas. Growing investments by governments and pharmaceutical companies for the development of innovative treatments like antifibrotic agents.
Moreover, raising awareness about rare diseases means finding ways to cure diseases and prepositions. Growing focus on health and wellness, as well as government and regulatory initiatives for healthcare R&D and pharmaceutical sectors, are expected to boost the scleroderma therapeutics market in the upcoming period. Ongoing investments for stem cell and gene researchers are projected to develop cutting-edge solutions for scleroderma. Additionally, ongoing developments in innovative therapies and technology are likely to shed light on the industry's growth.
What is the use of AI in the scleroderma therapeutics industry?
Artificial intelligence is taking the growth of the scleroderma therapeutics market to light by providing disease monitoring and diagnosis abilities. AI can analyze large databases and previous discoveries, which makes it easier for researchers to understand previous theories and reduce time investments. AI is able to provide all optimizing management processes as well as cost and time associated with research and manufacturing, which helps to build a better outlook plan.
AI allows for the generation of patient history, medical conditions, and genetic profiles, which helps to understand targeted treatments, and it becomes easier to develop effective treatments in the scleroderma therapeutics market. It is able to provide personalized medicine solutions by analyzing patient-relevant data and actual needs. The utilization of AI is making it easier to understand the manifestation and progression of the disease in the patient body. For instance, the utilization of AI in high-resolution computed tomography (HRCT) for treatments of systemic sclerosis is done with an understanding of lung disease. The benefits of AI in streamlining clinical trials, minimizing cost, and developing novel therapies are increasing the adoption of AI in the research and pharmaceutical sectors.
Scleroderma Therapeutics Market Growth Factors
- The rising prevalence of scleroderma disease in regions like North America and Europe is driving the growth of the market.
- Aging populations are the key factors contributing to the market growth due to the growing prevalence of disease among the elderly population and demands for scleroderma therapeutics.
- Expanding urbanization and changing lifestyles are the main reasons for inviting rare diseases like scleroderma.
- The increased awareness of disease among patients and healthcare professionals is shifting market expansions.
- Growing government focus and initiatives toward healthcare and the R&D sector are encouraging the development of innovative treatments, leading to boosting the scleroderma therapeutics market expansion.
- Growing pharmaceutical investments for research and development of novel therapies are highlighting the market potential.
- The expansion of treatment options, including oral, injectable, and topical, is making it easy to adopt novel advanced treatments.
- The adoption of advanced technologies like biomarker testing, imaging, 3D printing, and stem cells is holding market potential.
Scleroderma Therapeutics Market Outlook
Between 2025 and 2030, this market is expected to rise significantly due to several government initiatives aimed at developing the healthcare sector, coupled with rapid investment by market players for opening up new production centres to increase the production of therapies for treating scleroderma.
Numerous market players are actively entering this market, drawn by collaborations, R&D, and business expansions. Several therapeutics companies, such as Celgene Corporation, Argentis Pharmaceuticals, LLC, Emerald Health Pharmaceuticals, and others, have started investing rapidly in developing advanced treatments for treating scleroderma.
Various startup brands are engaged in developing advanced therapeutics across the globe. The prominent startup companies dealing in scleroderma therapeutics consists of Tempus, ElevateBio, Kaia Health, and some others.
Market Scope
| Report Coverage | Details |
| Market Size by 2026 | USD 2.88 Billion |
| Market Size in 2025 | USD 2.74 Billion |
| Market Size in 2035 | USD 4.48 Billion |
| Market Growth Rate from 2026 to 2035 | CAGR of 5.07% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Drug Class, Indication, and Regions. |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. |
Market Dynamics
Drivers
Increased research and development investments
The increasing prevalence of rare and critical diseases is prompting government and non-government bodies to prioritize investments in research and development of novel scleroderma therapeutics market solutions. Moreover, pharmaceutical biotechnology companies are also investing in research and development of cutting-edge treatments. With growing awareness of the disease among medical professionals and patients, the demand for effective treatment options has increased, leading to the encouraging development of innovative solutions.
Investment in research and development has led to the delivery of advanced therapies like immunosuppressants, antifibrotic agents, biologics, gene therapies, stem cells, and other small molecules, which help to manage the disease progress and also help to boost the immunity power of patients in the scleroderma therapeutics market. Moreover, the growing collaboration between pharmaceutical and biotechnology industries with universities and research teams is encouraging government and regulatory frameworks for further investments in the sectors.
Restraints
Limited understanding of scleroderma's pathophysiology
The limited understanding of pathophysiology is still a major restraint for the growth of the scleroderma therapeutics market. It is hard to identify effective targets to understand the diagnostics due to the involvement of immune cells by sclerodermas. Additionally, disease involves multiple pathways, which makes it hard for professionals to understand which pathway they should target. The development of antifibrotic treatments is still to be done due to a limited understanding of the mechanism of fibrosis. In recent studies, histopathological changes have been seen, which makes it more critical to understand the mechanisms of fibrosis. The lack of understanding of pathophysiology is hampering the development of effective treatments.
Opportunities
Developments of new biomarkers
The developments of novel biomarkers, like genetic, protein, and imaging biomarkers, hold potential in the scleroderma therapeutics market. The need for early detection and personalized treatments is leading to the development of genetic biomarkers. Similarly, protein biomarkers are being developed to monitor disease progress and response to treatments in the patient's body. Imaging biomarkers are important to assess skin and internal organ involvements. Imaging biomarkers like ultrasound and Magnetic Resonance Imaging are in high demand.
Biomarkers not only help to understand disease conditions and treatment processes but also help to develop novel scleroderma therapeutics market solutions. The need to improve patient outcomes is driving demands for biomarkers. Moreover, the growing investment by pharmaceutical and biotechnology companies in biomarker research and development is likely to transform the market. Additionally, government funding, support, and academic researchers are likely to contribute to the adoption and development of novel biomarkers to enhance treatment options for scleroderma.
Segment Insights
Drug class insights
The immunosuppressors segment contributed the highest share of the scleroderma therapeutics market in 2025. The immunosuppressors segment is leading the market due to its high demand for managing systemic scleroderma by suppressing the overactive immune response. Immunosuppressors help to reduce inflammation and fibrosis. Ongoing innovations in biologics and small-molecule immunosuppressors are the key reasons behind the expanding growth of the segment. Due to the ability to drive control over manifestations of scleroderma, like lung fibrosis and renal involvement, healthcare providers have increased the adoption of immunosuppressors in recent years. With the growing prevalence of scleroderma and the need for effective therapies in reducing disease progression, the segment is expected to continue dominating the market. Moreover, growing regulatory support and investments are driving the segment's success.
In October 2024, the FDA of an amendment to its IND application gave clearance to Adicet Bio's ADI-100, which is an investigational allogeneic CAR-engineered gamma delta T-cell therapy, recently being evaluated in a phase 1 clinical trial (NCT06375993) for lupus nephritis (LN) for patients with IIM and SPS into the trial.
On the other hand, the endothelin receptor antagonist segment is expected to grow at a significant CAGR in the scleroderma therapeutics market during the forecast period. The growth of the segment is anticipated due to its growing adoption in healthcare due to its ability to block the actions of endothelin, a potent blood vessel constrictor, which develops the scleroderma condition. The receptor also effectively manages pulmonary arterial hypertension (PAH), a common complication of scleroderma. The endothelin receptor antagonist is being highlighted due to its ability to improve patients' exercise capacity and reduce the slow progression of disease in patients who are suffering from PAH. The adoption of some endothelin receptor antagonists like Bosentan, Ambrisentan, and Macitentan is high. With the growing need for the management of PAH and other complications of scleroderma, the adoption of segments is expected to boost in the forecast period.
Indication insights
The systemic scleroderma segment held the dominant share of the scleroderma therapeutics market in 2025. The segment growth is attributed to higher prevalence and greater severity of systemic scleroderma. The increased awareness and advanced diagnosis are driving the growth of the segment. Moreover, an aging population is contributing to the expansion. Systemic scleroderma improves morbidity and mortality by directly affecting inner organs like lungs and kidneys. The growing investments of pharmaceutical companies in the development of innovative treatments for systemic scleroderma.
- In March 2024, Cabaletta Bio received the FDA-granted Orphan Drug Designation (ODD) for its CABA-201, an investigational therapy for scleroderma. CABA-201 is being developed as a treatment for autoimmune diseases, and it is a CD19-CAR T cell therapy.
- In November 2024, the Scleroderma Research Foundation (SRF) published a peer-reviewed article on CONQUEST, a highly innovative platform clinical study that accelerates the development of new therapies for systemic sclerosis. CONQUEST focuses on early, active systemic sclerosis with interstitial lung disease (SSc-ILD), a serious complication that is the leading cause of death in scleroderma.
However, the localized scleroderma segment is anticipated to grow at the highest CAGR in the scleroderma therapeutics market during the forecast period. Localized scleroderma has properties of limited skin involvement. Localized scleroderma can mostly be treated by utilizing topical medications and phototherapy. Continuous innovations in the development of novel treatments for localized scleroderma, like biologics and small molecules, are fueling the segment expansion. Additionally, the ability of localized scleroderma to be cured by using systemic immunosuppressive therapy drives cutting-edge research in the treatments.
Regional Insights
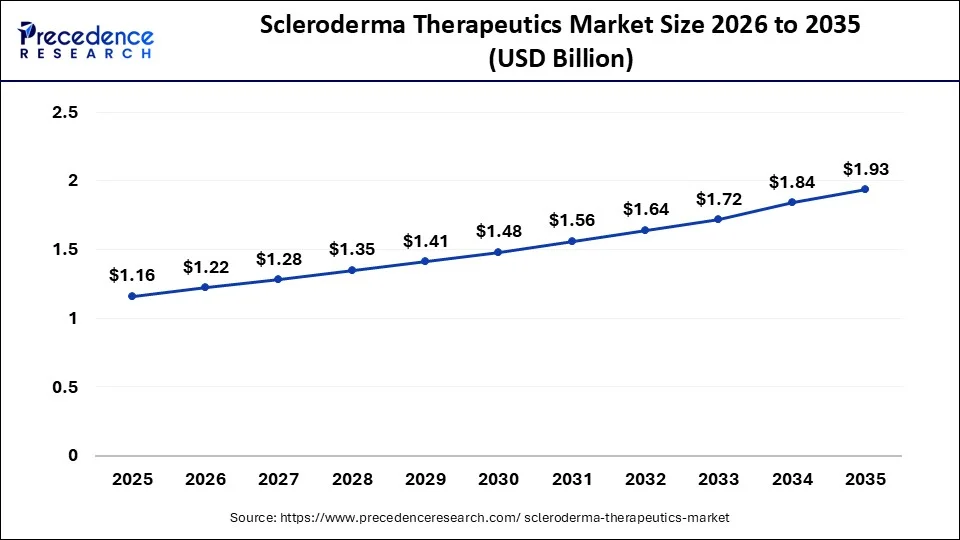
The U.S. scleroderma therapeutics market size is exhibited at USD 1.16 billion in 2025 and is projected to be worth around USD 1.93 billion by 2035, growing at a CAGR of 5.22% from 2026 to 2035.
North America dominated the scleroderma therapeutics market in 2025 due to the high prevalence of the disease. North America is well known for the availability of established healthcare and pharmaceutical sectors. The growing prevalence of scleroderma disease in the region is driving the focus of regulatory frameworks to support and invest in research and development of novel treatment solutions. The region is contributing to research and development firms for innovative treatment solutions. Numerous research institutes and universities are driving innovation in the region.
- In January 2025, a research team at the cross-disciplinary University of Alberta developed a biological marker for scleroderma, which can predict which patients are likely to develop severe disease and could also lead to new treatments for the disease.
The United States is leading the North American scleroderma therapeutics market due to the high prevalence of scleroderma disease in an aging population and the country's well-established healthcare sector. However, Canada witnesses significant growth in the market with a rapidly growing prevalence of the disease. Moreover, the presence of a regulatory framework like the Food and Drug Administration (FDA) is playing a crucial role in the regional market expansion.
- According to NCBI, scleroderma has affected about 17,000 people in Canada, and about 40% of patients have died within five years of diagnosis in the country. Government and non-government bodies are investing in research and development, leading to boosting the country's market.
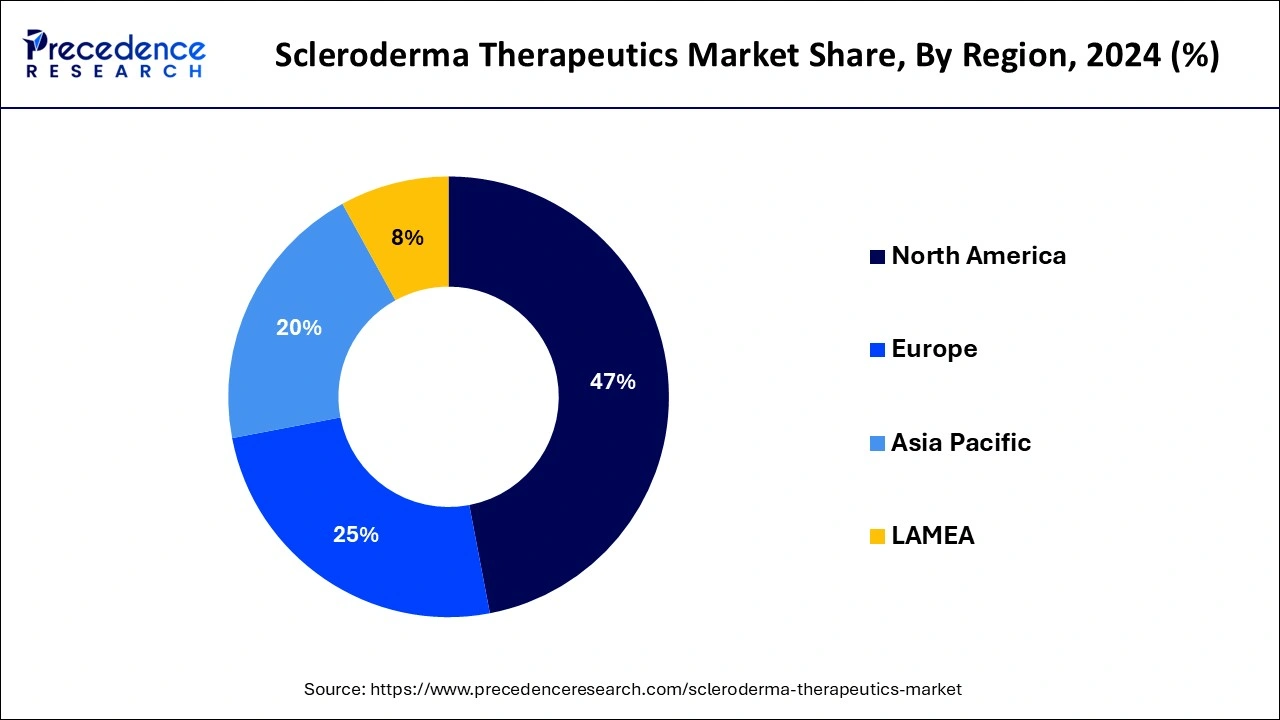
Asia Pacific is projected to host the fastest-growing scleroderma therapeutics market in the forecast period due to an increase in investment in the research and development sector. The surge of investment by countries in Japan, China, and India for new drug candidates, innovative therapies, and developments of advanced technologies is contributing to the growth of the market in the region. The prevalence of scleroderma in Asia has increased, leading to demand for effective treatments. Collaborations between hospitals and medical centers are generating room for market growth.
Japan is leading the Asia Pacific scleroderma therapeutics market due to the country's government focus and investment in the healthcare sector. However, India is anticipated to witness significant growth in the market due to increased patient populations and government investments in healthcare expenditure. Moreover, government support and initiatives to encourage R&D activities in pharmaceutical sectors for the development of orphan drugs are playing a crucial role in the market expansion.
- In February 2025, Certa Therapeutics (Certa), a biotechnology company developing innovative precision therapies for patients with inflammatory and fibrotic diseases, received the U.S. Food and Drug Administration (FDA) approval of Fast Track Designation for treatment of systemic sclerosis (scleroderma) called investigational therapy FT011, which had previously granted Orphan Drug Designation.
Europe held a significant share of the market. The increasing cases of skin diseases in different nations, including Germany, France, Italy, the UK, and some others, have boosted the market expansion. Also, numerous government initiatives aimed at developing the healthcare sector are expected to accelerate the growth of the scleroderma therapeutics market in this region.
Latin America held a considerable share of the industry. The rapid expansion of the biopharma sector in numerous countries such as Brazil, Argentina, Peru, Venezuela, and some others has driven the market growth. Additionally, rapid investment by market players for opening up new medicine production centers is expected to drive the growth of the scleroderma therapeutics market in this region.
The Middle East and Africa held a notable share of the market. The growing demand for immunosuppressors from the derma sector in several nations, including the UAE, Saudi Arabia, South Africa, and some others, has propelled the market expansion. Also, rising investment by pharma companies for opening up new research and development centers is expected to propel the growth of the scleroderma therapeutics market in this region.
Scleroderma Therapeutics Market Companies
F. Hoffmann-La Roche AG, or Roche, is a Swiss multinational healthcare company that operates in the Pharmaceuticals and Diagnostics divisions. It develops and manufactures medicines and diagnostics to treat major diseases, including cancer, autoimmune, and central nervous system disorders.
Squibb Company: Bristol-Myers Squibb (BMS) is a global biopharmaceutical company focused on discovering, developing, and delivering innovative medicines to help patients with serious diseases. BMS emphasizes science, patient-centricity, ethical conduct, and global corporate responsibility.
Calliditas Therapeutics AB is a commercial-stage biopharmaceutical company based in Stockholm, Sweden, that develops treatments for rare, orphan diseases with a focus on renal and hepatic conditions. The company's goals include improving the lives of patients and expanding treatment options for diseases with few or no existing approved treatments.
Celgene Corporation is a biopharmaceutical company focused on cancer and inflammatory diseases. This company specializes in discovering, developing, and commercializing therapies for cancer and immune-inflammatory diseases, using a science-based approach of gene and protein regulation.
Argentis Pharmaceuticals is a biopharmaceutical company focused on developing orphan drugs for autoimmune diseases, particularly late-stage diffuse systemic scleroderma (dcSSc). This company's primary focus is on developing treatments for autoimmune diseases, with a specific emphasis on dcSSc.
Emerald Health Pharmaceuticals was a clinical-stage biotechnology company focused on developing cannabinoid-derived drugs for neurodegenerative and autoimmune diseases. This company is engaged in developing proprietary cannabinoid-derived medicines for central nervous system (CNS), autoimmune, inflammatory, and fibrotic diseases.
Kadmon Holdings was a biopharmaceutical company focused on developing therapies for inflammatory and fibrotic diseases, immuno-oncology, and other conditions. It specializes in discovering, developing, and commercializing small molecules and biologics for diseases with significant unmet medical needs, particularly inflammatory and fibrotic diseases.

Other Major Key Players
- Bayer AG
- Sanofi
- Cytori Therapeutics Inc.
- Akashi Therapeutics
- Prometic Life Sciences
Latest Announcements by Industry Leaders
- In November 2024, Dr. Luke Evnin, Chairman of the Board for the SPF, talked about the Scleroderma Research Foundation's launching of CONQUEST; “The introduction of the CONQUEST trial reflects the spectacular milestone in companies' efforts to improve scleroderma research.”
- In September 2024,” Darren Kelly, PhD, CEO, founder, and managing director of Certa, talked in a company press release about receiving the INN name for Certa Therapeutics' oral drug candidate; “Getting an INN for companies like oral, lead drug candidate as asengeprast, is the most important step in the development of this novel important therapy.”
Recent Developments in Scleroderma Therapeutics
- In November 2025, Sandoz launched TYRUKO. TYRUKO is an advanced biosimilar designed for treating patients suffering from multiple sclerosis.
(Source: www.sandoz.com) - In June 2025, Cabaletta Bio, Inc. launched a new range of medicines for treating scleroderma. This new range of medication is designed for the consumers of the U.S.
(Source: www.cabalettabio.com)
Segments Covered in the Report
By Drug Class
- Immunosuppressors
- Phosphodiesterase 5 inhibitors – PHA
- Endothelin Receptor Antagonists
- Prostacyclin Analogues
- Calcium Channel Blockers
- Analgesics
- Others
By Indication
- Systemic Scleroderma
- Morphea
- Linear
- Localized Scleroderma
- Diffuse Systemic Sclerosis
- Limited Cutaneous Systemic Sclerosis Syndrome
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting