Autologous Stem Cell and Non-Stem Cell Based Therapies Market Size and Forecast 2025 to 2034
The global autologous stem cell and non-stem cell based therapies market size accounted for USD 8.64 billion in 2024 and is predicted to increase from USD 9.64 billion in 2025 to approximately USD 25.78 billion by 2034, expanding at a CAGR of 11.55% from 2025 to 2034.The market is experiencing significant growth due to the rising prevalence of chronic and degenerative diseases, advancements in regenerative medicine, and the demand for personalized treatment approaches. Increased clinical research and a wider range of applications in orthopedic, cardiovascular, and neurological disorders are expected to further accelerate market growth.
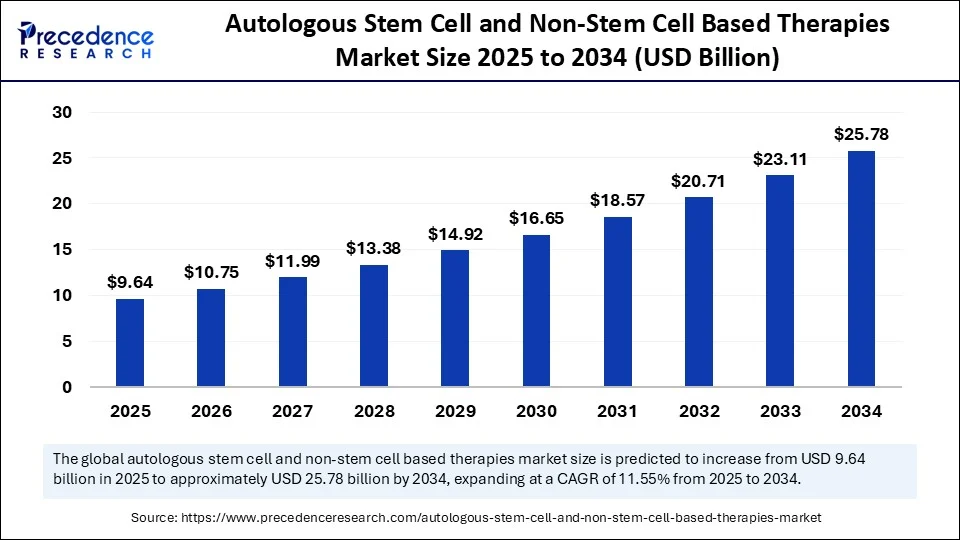
Autologous Stem Cell and Non-Stem Cell Based Therapies Market Key Takeaways
- In terms of revenue, the global autologous stem cell and non-stem cell based therapies market was valued at USD 8.64 billion in 2024.
- It is projected to reach USD 25.78 billion by 2034.
- The market is expected to grow at a CAGR of 11.55% from 2025 to 2034.
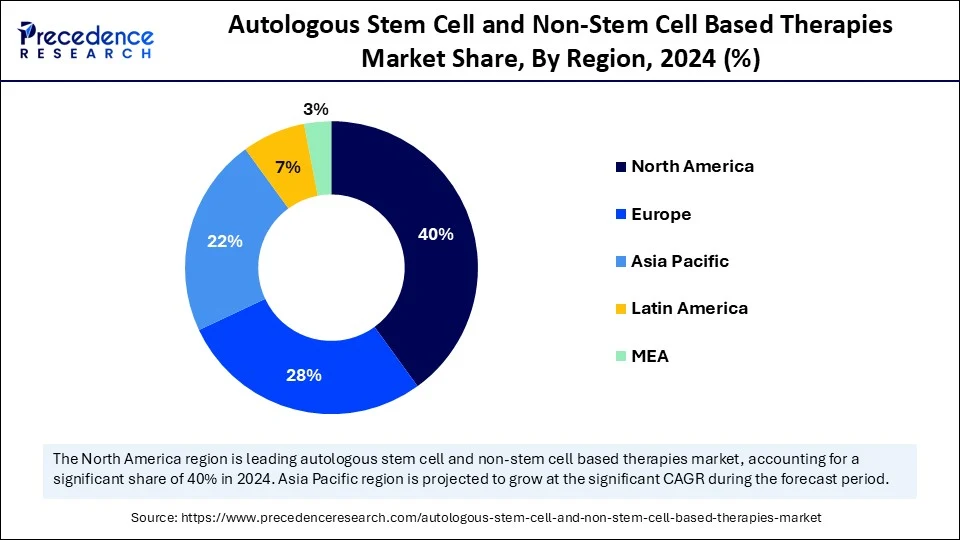
- North America dominated the global autologous stem cell and non-stem cell based therapies market with the largest share of 40% in 2024.
- Asia Pacific is anticipated to grow at a significant CAGR from 2025 to 2034.
- By therapy type, the autologous stem cell-based therapies segment captured the largest revenue share of 65% in 2024.
- By therapy type, the autologous non-stem cell-based therapies segment is anticipated to grow at a significant CAGR from 2025 to 2034.
- By application, the oncology segment contributed the highest market share of 30% in 2024.
- By application, the neurology segment is anticipated to grow at a significant CAGR from 2025 to 2034.
- By end user, the hospitals and clinics segment generated the major market share of 55% in 2024.
- By end user, the specialized cell therapy centers segment is expected to witness the fastest CAGR during the foreseeable period.
How Can AI Impact the Autologous Stem Cell and Non-Stem Cell Based Therapies Market?
Artificial intelligence (AI) is also transforming the autologous stem cell and non-stem cell based therapies market by facilitating personalized treatments, improving cell characterization, optimizing differentiation, and enhancing diagnostic capabilities. In both autologous (patient-derived) and non-autologous (donor-derived) cell therapies, AI analyzes large datasets to predict patient-specific outcomes, identify optimal cell products, and accelerate the development of new regenerative medicine approaches for conditions like diabetes and liver disease. AI enhances advanced microscopy and other imaging techniques, enabling precise, non-invasive, and quantitative live cell analysis to support early diagnosis and treatment monitoring.
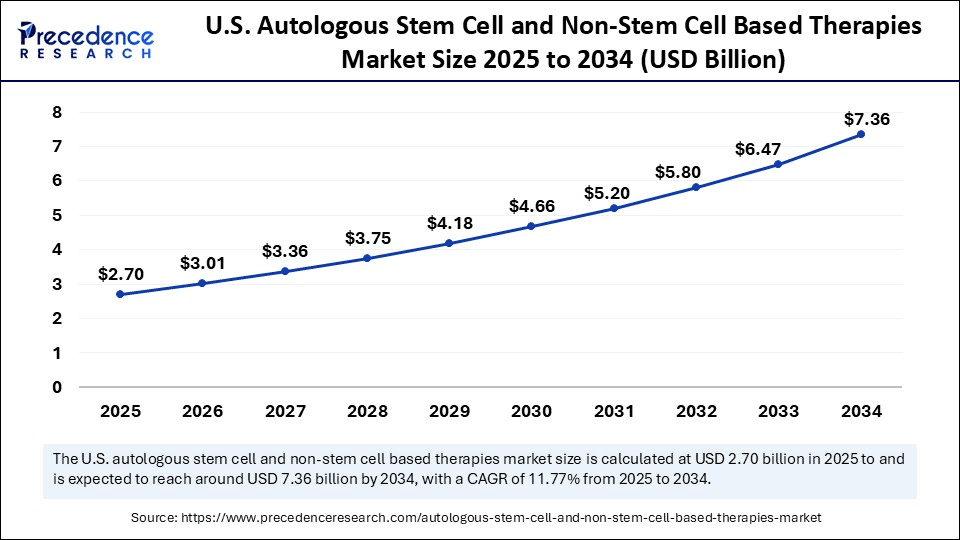
U.S. Autologous Stem Cell and Non-Stem Cell Based Therapies Market Size and Growth 2025 to 2034
The U.S. autologous stem cell and non-stem cell based therapies market size was exhibited at USD 2.42 billion in 2024 and is projected to be worth around USD 7.36 billion by 2034, growing at a CAGR of 11.77% from 2025 to 2034.
How Did North America Lead the Autologous Stem Cell and Non-Stem Cell Based Therapies Market in 2024?
North America led the global market in 2024. This is mainly due to its well-developed healthcare infrastructure, strong focus on research and development, and a robust regulatory framework that encourages innovation. A reliable healthcare system supports the delivery and growth of complex cell-based therapies. The rising number of chronic illnesses, such as cardiovascular disorders, cancer, and neurodegenerative diseases, creates a high demand for innovative stem cell therapies that can repair or replace damaged cells. Additionally, the presence of key players like Bristol-Myers Squibb, Gilead Sciences, and Novartis, along with a supportive regulatory environment, promotes the development and commercialization of these treatments.
In June 2025, the U.S. FDA approved label updates for two CAR T-cell therapies: Breyanzi (liso-cel) for large B-cell lymphoma and Abecma (ide-cel) for multiple myeloma. This change aims to increase access to cell therapies, which currently reach only 20% of eligible patients. The updates reduce specific patient monitoring requirements and eliminate REMS programs, reflecting growing confidence in the safety of these therapies, benefitting more patients with blood cancers. (Source: https://www.curetoday.com)
The U.S. Autologous Stem Cell and Non-Stem Cell Based Therapies Market Trends
The U.S. plays a significant and evolving role in the global market, driven by substantial investments in research and development, a robust healthcare infrastructure, and a supportive regulatory framework, including expedited approval pathways like the regenerative medicine advanced therapy designation. The U.S. also leads in the number of clinical trials and FDA approvals for these innovative therapies, particularly in the rapidly expanding oncology segment, such as CAR-T cell therapies for blood cancers. Furthermore, the presence of key players, strategic collaborations, and technological advancements reinforces the U.S.'s position as a hub for innovation and progress in personalized medicine.
Canada Autologous Stem Cell and Non-Stem Cell Based Therapies Market Trends
Canada also plays a distinct role in the market. The country is characterized by strong research initiatives and a high growth rate. Canada benefits from robust public-private partnerships, exemplified by the Stem Cell Network and collaborations like the one between Aspect Biosystems and Novo Nordisk, which drive innovation and biomanufacturing capabilities. Although the regulatory framework is flexible yet stringent, allowing for the development of novel cell and gene therapies, Health Canada must still tackle challenges such as unregulated clinics offering unproven treatments and the need to expedite product commercialization.
How Will Asia Pacific be Considered the Fastest-Growing Region in the Autologous Stem Cell and Non-Stem Cell Based Therapies Market in 2024?
Asia Pacific is expected to witness the fastest growth during the forecast period. This is primarily due to its large population, the rising prevalence of chronic diseases, increasing government support for regenerative medicine, and favorable regulatory environments in countries like Japan. Additionally, advancements in research and development and medical infrastructure, including medical tourism, are contributing to this growth. Countries such as Japan and South Korea have established supportive policies, like Japan's Regenerative Medicine Promotion Act, to streamline the approval process for regenerative medicines and therapies. Emerging countries like India and China are becoming hubs for medical tourism, attracting patients worldwide seeking stem cell-based treatments, along with the development of GMP-certified facilities for cell therapy production.
Market Overview
The autologous stem cell and non-stem cell based therapies market encompasses the field of regenerative medicine and therapeutic interventions in which cells or tissues are sourced from the patient's own body (autologous source) and reintroduced to repair, replace, or restore damaged tissues. This market involves both stem cell-based therapies, such as mesenchymal and hematopoietic stem cells, as well as non-stem cell-based therapies, including immune cells, skin cells, and platelets.
Applications of these therapies span various medical fields, including oncology, orthopedics, cardiology, neurology, and dermatology. Currently, the market is experiencing growth due to the increasing number of regulatory approvals for advanced therapies, heightened research and development efforts, investments in personalized medicine, the growing prevalence of chronic diseases such as cancer and autoimmune disorders, and the expanding elderly population.
What Are the Key Trends in the Autologous Stem Cell and Non-Stem Cell Based Therapies Market?
- Demand for Personalized and Targeted Therapies: Autologous therapies are uniquely suited to meet the demand for personalized treatment, as they are tailored to the specific needs of individual patients.
- Advances in Regenerative Medicine: Progress in techniques for cell isolation, expansion, and differentiation has improved the safety and effectiveness of stem cell and other cell-based therapies, thus promoting their use and driving their development and commercialization.
- Rising Research and Development: Significant investments in research and development for autologous therapeutics, including non-stem cell-based approaches like CAR T-cells, are expanding treatment options and driving market growth.
- Regulatory Approvals and Initiatives: Favorable regulatory approvals and government initiatives aimed at new autologous treatments, along with increased investment in research and development and financial support, are accelerating market expansion.
- Expanding Geriatric Population: The growth of the global geriatric population is also contributing to market growth, as older individuals often have a higher prevalence of chronic diseases.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 25.78 Billion |
| Market Size in 2025 | USD 9.64 Billion |
| Market Size in 2024 | USD 8.64 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 11.55% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Therapy Type, End User, Application, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising Incidence of Chronic and Degenerative Diseases
The primary driver of the autologous stem cell and non-stem cell based therapies market is the rising incidence of chronic and degenerative diseases, including cancer, diabetes, cardiovascular disorders, and neurological conditions. This upward trend necessitates advanced treatments and drives demand for regenerative therapies. Cell therapies are expanding their applications beyond initial uses in hematologic cancers and orthopedic injuries to address a broader range of conditions. Increased investment in research and development for cell therapy fuels innovation, leading to new therapeutic approaches and further market expansion.
Restraint
High Cost of Production and Treatment
A significant restraint in the autologous stem cell and non-stem cell based therapies market is the high cost of production and treatment associated with personalized autologous therapies. These costs arise from the complex manufacturing process, regulatory challenges, and lack of economies of scale. Each autologous treatment is customized for individual patients, necessitating extensive cell separation, processing, and modification. This complexity drives up manufacturing costs and complicates large-scale production, making it difficult to reduce prices through mass production.
Opportunity
Integration With Advanced Biotechnologies
Looking ahead, a key opportunity in the market lies in integrating advanced biotechnologies, such as gene editing (CRISPR), synthetic biology, and exosome-based therapeutics. These technologies aim to create more precise, scalable, and effective personalized treatments for chronic and degenerative diseases, including cancer and autoimmune disorders. The overarching trend is to leverage advanced technologies to develop therapies tailored to individual patient needs, moving beyond generic approaches to target specific disease mechanisms.
Therapy Type Insights
What made the Autologous Stem Cell-Based Therapies Segment Lead the Autologous Stem Cell and Non-Stem Cell Based Therapies Market in 2024?
The autologous stem cell-based therapies segment dominated the market in 2024. This is mainly because of reduced risks of immune rejection and ethical concerns, the increasing prevalence of chronic and degenerative diseases, and the development of personalized treatments like CAR T-cell therapies for cancer. Using a patient's own cells for regeneration and repair makes these therapies safer and more preferred, bypassing the ethical issues linked to human embryonic stem cells. Advances in cancer treatment are helping this market grow and encouraging adoption. These cells have remarkable abilities to proliferate and differentiate, making them ideal for regenerating damaged tissues and treating conditions caused by cellular degeneration.
The autologous non-stem cell-based therapies segment is experiencing the fastest market growth. This is mainly because of more regulatory approvals for therapies like CAR T-cell therapy, expanding uses beyond cancer to autoimmune and solid tumor treatments, increasing patient awareness, and the potential of new therapies like Platelet-Rich Plasma (PRP) and exosomes. This success is boosting research into new applications for solid tumors and autoimmune diseases, reaching a larger patient group. These therapies offer regenerative and reparative potential for chronic conditions once thought untreatable. Progress in regenerative medicine, along with regulatory support and rising demand for personalized treatments, is fueling this segment's rapid expansion.
Application Insights
How Did the Oncology Segment Dominate the Autologous Stem Cell and Non-Stem Cell Based Therapies Market in 2024?
The oncology segment led the market in 2024. This growth mainly results from the increasing number of approved therapies like CAR T-cell therapy and tumor-infiltrating lymphocyte (TIL) therapy, which use the body's own cells to fight cancer. The rising prevalence of various cancers and the promise of personalized, effective treatments drive extensive research and development, leading to a larger market share compared to other therapy areas, as autologous therapies are ideal for cancer treatments because they provide personalized solutions with lower rejection risk. The urgent need for effective cancer treatments has driven significant investment and innovation, resulting in a pipeline of new therapies and more treatment options.
The neurology segment is expected to grow the fastest during the forecast period. This is mainly due to the rising prevalence of neurodegenerative and chronic neurological disorders like Alzheimer's, Parkinson's, and ALS, combined with the regenerative potential of cell therapies to repair damaged neural tissue. Autologous therapies have the potential to regenerate neural tissue, which addresses a critical unmet need in treating neurological conditions. Advances in isolating, differentiating, and expanding cells, along with innovations in non-stem cell therapies like exosomes, are improving the safety and effectiveness of these treatments for neurological disorders, enabling new applications.
End User Insights
Why Did the Hospitals and Clinics Segment Dominate the Autologous Stem Cell and Non-Stem Cell Based Therapies Market in 2024?
The hospitals and clinics segment maintained a leading position in the market in 2024. This is primarily because they are central to patient care, have the necessary infrastructure for complex procedures, and are equipped to handle the regenerative and personalized nature of these advanced treatments, thus driving demand. Patients with chronic conditions prefer these therapies at hospitals for comprehensive care and specialized treatment. Hospitals and clinics are also best equipped to meet strict regulatory requirements and quality control standards necessary for producing and administering cell-based therapies.
The specialized cell therapy centers segment is expected to see the fastest growth in the market. This growth stems from the rising prevalence of chronic diseases, which increases demand for regenerative and personalized treatments using a patient's own cells. Favorable regulatory approvals for therapies like CAR T-cell treatments for cancer, along with technological improvements in cell isolation, expansion, and engineering, are major factors. Ongoing advancements in cell processing and engineering techniques, including advanced cell isolation, expansion, and differentiation methods, enhance the safety and effectiveness of these therapies to promote tissue repair and regeneration, which fuels growth.
Value Chain Analysis
- R&D
The R&D focuses on developing personalized treatments that use a patient's own cells to repair and regenerate damaged tissue. Key areas of development include CAR T-cell therapies for cancer and stem cell therapies for a range of conditions, like cardiovascular and neurodegenerative diseases.
Key Players: Bristol-Myers Squibb Company, Gilead Sciences, Inc., Novartis AG, Johnson & Johnson, Caladrius Biosciences Inc.
- Clinical Trials and Regulatory Approvals
This involves developing, testing, and gaining regulatory approval for treatments that use a patient's own cells. Ongoing R&D and growing approvals drive market expansion in oncology and other areas like autoimmune diseases.
Key Players: Novartis, Johnson & Johnson, Iovance Biotherapeutics, Gilead Sciences, Dendreon Corporation
- Formulation and Final Dosage Preparation
The formulation and final dosage preparation involve the complex process of turning a patient's own cells into a final, viable treatment owing to increasing demand for personalized medicine and rising regulatory approvals, particularly for cancer therapies like CAR T-cells.
Key Players: Novartis AG, Charles River Laboratories, Bristol-Myers Squibb, Thermo Fisher Scientific, Johnson & Johnson
- Packaging and Serialization
This focuses on the critical, patient-specific logistics required for these treatments. This involves developing specialized packaging, often with cryogenic capabilities, and robust serialization systems to ensure a secure chain of identity from the patient back to the patient.
Key Players: Thermo Fisher Scientific, WuXi AppTec, Lonza Group, QuickSTAT, Cryoport Systems
- Distribution to Hospitals, Pharmacies
This covers the complex logistics of delivering highly personalized cell-based treatments to healthcare providers. These therapies are driven by rising cancer and chronic disease rates, require strict handling due to their time-sensitive and patient-specific nature facilities rather than a standard drug supply chain.
Key Players: Vericel Corporation, Dendreon Pharmaceuticals LLC, Gilead Sciences, Inc., Catalent, Inc., Charles River Laboratories
- Patient Support and Services
This provides vital assistance to patients undergoing complex regenerative treatments using their own cells. Services include logistical coordination, financial navigation for high-cost therapies, and patient education for treatments like CAR T-cell therapy for personalized medicine and advancements in cell technologies.
Key Players: Gilead Sciences, Inc., Novartis AG, Bristol-Myers Squibb Company, ProPharma Group, Cryoport, Inc.
Autologous Stem Cell and Non-Stem Cell Based Therapies Market Companies

- Vericel Corporation
- Holostem Terapie Avanzate
- Anterogen Co. Ltd.
- BrainStorm Cell Therapeutics
- Fibrocell Science
- Cytori Therapeutics
- TiGenix (Takeda)
- CORESTEM Inc.
- Smith & Nephew (via Osiris Therapeutics)
- Pluristem Therapeutics
- ThermoGenesis Holdings
- Regenexx
- Gilead Sciences
- Novartis
- Fate Therapeutics
- Medeor Therapeutics
- Lineage Cell Therapeutics
- GenCure
- Aastrom Biosciences (Vericel)
- Celgene
Leaders' Announcements
- In January 2024, STEMCELL Technologies acquired Propagenix Inc., a company focused on regenerative medicine technologies. This acquisition is crucial for our growth and can advance treatments for various disorders, stated STEMCELL CEO Dr. Allen Eaves. Propagenix's technologies will support clinical applications of engineered tissues.
(Source: https://www.stemcell.com) - In November 2023, Legend Biotech announced an exclusive License Agreement with Novartis for certain CAR-T cell therapies targeting DLL3, including its candidate, LB2102. This agreement allows Novartis to develop and commercialize these therapies, marking the use of its T-Charge™ platform on solid tumors. They believe LB2102 has innovative features that enhance anti-tumor activity, remarked Guowei Fang, Chief Scientific Officer of Legend Biotech.(Source: https://investors.legendbiotech.com)
Recent Developments
- In June 2025, BioRestorative Therapies, Inc. promised preliminary blinded data from its Phase 2 clinical trial of BRTX-100, an autologous stem cell therapy for chronic lumbar disc disease (cLDD). The U.S. FDA requires over 30% improvement in the Oswestry Disability Index and Visual Analog Scale for trial progression. The CEO, Lance Alstodt, emphasized the therapy's potential for addressing chronic lower back pain. (Source: https://www.prnewswire.com)
- In April 2024, Vertex Pharmaceuticals obtained an exclusive license for TreeFrog Therapeutics' C-Stem™ technology to enhance the production of cell therapies for type 1 diabetes (T1D). Vertex plans to scale up this technology to generate large amounts of fully differentiated cells for T1D treatments. Their goal is to transform T1D treatment, remarked by Morrey Atkinson, EVP at Vertex. (Source: https://ir.biorestorative.com)
Segments Covered in the Report
By Therapy Type
- Autologous Stem Cell-Based Therapies
- Mesenchymal Stem Cells (MSCs)
- Hematopoietic Stem Cells (HSCs)
- Neural Stem Cells
- Epithelial Stem Cells
- Others
- Autologous Non-Stem Cell-Based Therapies
- Autologous Chondrocytes
- Autologous Fibroblasts
- Immune Cells (e.g., T-cells, NK cells, dendritic cells)
- Platelet-Rich Plasma (PRP)
- Others
By Application
- Oncology
- Orthopedics & Musculoskeletal Disorders
- Cardiology
- Neurology
- Dermatology & Wound Healing
- Autoimmune Disorders
- Others
By End User
- Hospitals & Clinics
- Specialized Cell Therapy Centers
- Academic & Research Institutes
- Others
By Region
- North America
- Europe
- Asia Pacific
- South America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting