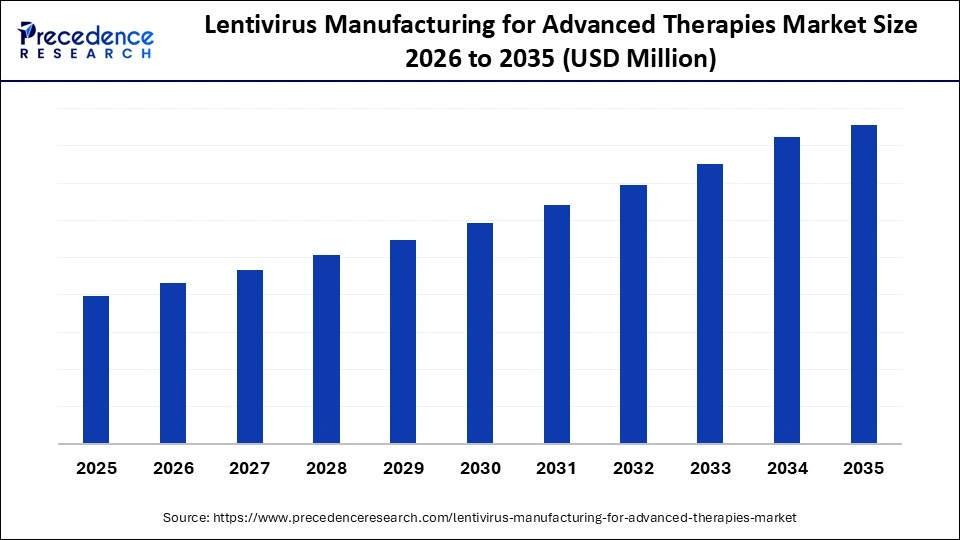
What is the Lentivirus Manufacturing for Advanced Therapies Market Size?
The global lentivirus manufacturing for advanced therapies market is growing as demand rises for viral vectors used in gene therapy, cell therapy, and cutting-edge regenerative treatments.The expansion of gene therapy pipelines primarily drives the lentivirus manufacturing for advanced therapies market.
Market Highlights
- North America dominated the market, holding the largest market share of 42.40% in 2025.
- The Asia Pacific is expected to expand at the fastest CAGR of 13.5% between 2026 and 2035.
- By application, the gene therapy segment contributed the highest market share of 45.60% in 2025.
- By application, the cancer immunotherapy segment is projected to grow at a notable CAGR of 11.1% from 2026 to 2035.
- By end-user, the pharmaceutical & biotechnology companies segment captured the highest market share of 43.5% in 2025.
- By end-user, the research institutes & academic institutions segment is growing at a strong CAGR of 11.3% between 2026 and 2035.
- By scale of operation, the clinical / pilot-scale production segment held the major market share of 62.4% in 2025.
- By scale of operation, the commercial/large-scale production segment is set to grow at a healthy CAGR of 11.6% between 2026 and 2035.
Lentivirus Manufacturing for Advanced Therapies Market Overview
The lentivirus manufacturing for the advanced therapies market comprises the development and GMP-compliant production of lentiviral vectors used in gene and cell therapies. These vectors serve as essential delivery systems that transport therapeutic genes into target cells, resulting in stable, long-lasting expression. The market spans the full lifecycle of vector production. This begins with vector design, where developers optimize promoter selection, transgene configuration, and safety features to ensure high performance in clinical applications.
Upstream manufacturing includes cell culture, transfection, and vector generation using producer or packaging cell lines. Each step requires controlled bioreactor conditions, precise nutrient management, and validated transfection protocols to achieve high yield and consistent vector potency. Downstream processes follow with purification steps designed to remove impurities, host cell proteins, residual plasmid DNA, and other contaminants. Chromatography, filtration, and concentration methods are used to obtain clinical-grade material with defined purity and safety attributes.
The market also includes fill-finish operations, in which vectors are formulated, aliquoted, and packaged into vials under aseptic conditions. Quality control activities span the entire workflow and include assays for infectivity, transduction efficiency, sterility, identity, and genomic integration profiles. These processes ensure that vectors meet regulatory expectations for both clinical and commercial use.
How Are AI-Driven Innovations Reshaping the Lentivirus Manufacturing for Advanced Therapies Market?
In today's rapidly evolving technological landscape, the integration of artificial intelligence (AI) is a transformative force. It holds great potential to accelerate the growth of the lentivirus manufacturing for advanced therapies market by optimizing the entire workflow, from vector design to quality control. The integration of AI allows companies to make data-driven decisions. This leads to reducing CAR-T cell manufacturing timelines, lower costs, and greater patient access to advanced treatments. AI-integrated bioprocessing platforms enable real-time quality control, predictive analytics, and error detection. AI-based computational modeling is less expensive than traditional laboratory methods for optimizing an effective and safe gene delivery carrier.
Lentivirus Manufacturing for Advanced Therapies Market Outlook
Between 2026 and 2035, the industry is expected to experience accelerated growth. The market is driven by the growing number of cell and gene therapies, particularly for cancer treatment and genetic disorders, increasing outsourcing trends, and the growing demand for scalable & regulatory-compliant vector production.
Several leading players in the lentivirus manufacturing for advanced therapies market are increasingly focusing on expanding their global footprint through facility expansions, strategic partnerships, and acquisitions. For instance, in August 2024, Merck, a leading science and technology company, announced the closing of the transaction to acquire Mirus Bio for approximately USD 600 million. The acquisition is a strategic step towards Merck's ambition to offer solutions for every step of viral vector manufacturing. It also reinforces the company's commitment to supporting customers in advancing cell and gene therapies from preclinical through commercial production.
Major investors play a crucial role in the lentivirus manufacturing for the advanced therapies market by providing capital, expertise, and strategic direction needed to advance research and scale up production for cell and gene therapies. Both established biotech/pharmaceutical companies and specialized Contract Development and Manufacturing Organizations (CDMOs) are making these investments, often with financial backing from investment firms. In February 2025, Novartis in Slovenia officially opened the new viral vector production facility (VIFA One) in Menges.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Application, End-User, Scale of Operation, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Lentivirus Manufacturing for Advanced Therapies Market Segmental Insights
Application Insights
Gene Therapy: The gene therapy segment held the largest market share, at 45.6%, in 2025. The segment's growth is primarily driven by rising demand for effective treatments for cancer and genetic disorders, the growing number of clinical trials using LVs, and the need for scalable, effective manufacturing methods. Lentiviral vectors have emerged as crucial tools in gene therapy, offering new opportunities for treating a variety of genetic illnesses.
Cancer Immunotherapy: On the other hand, the cancer immunotherapy segment is expected to grow at a remarkable CAGR between 2026 and 2035. The segment's growth is driven by the rising effectiveness of lentiviral vectors for gene delivery in therapies, the expanding pipeline of cancer-focused gene therapies, and the integration of lentiviral vectors into cell-based immunotherapies, such as CAR-T cells. Cancer is the primary disease target for lentiviral vectors, owing to the increasing prevalence of various cancer types and a significant need for innovative therapies.
Scale of Operation Insights
Clinical / Pilot-Scale Production: The clinical / pilot-scale production segment contributed the highest revenue share in the lentivirus manufacturing for advanced therapies market, owing to the increasing need to optimize upstream and downstream such as purification, concentration, and formulation processes to improve overall yield and purity. Moreover, the expanding pipeline of gene and cell therapies in clinical trials, the increasing need for scalable and cost-effective manufacturing platforms for late-stage trials, and to meet strict regulatory requirements.
Commercial / Large-scale Production: On the other hand, the commercial/large-scale production segment is set to grow at the fastest CAGR of 11.6% between 2026 and 2035, owing to the increasing demand for advanced therapies, particularly gene and cell therapies, a rise in chronic diseases, and rapid technological advancements in manufacturing processes. Moreover, the rising number of clinical trials and regulatory approvals for lentivirus-based products, including those from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), is expected to drive the need for commercial-scale manufacturing.
End-User Insights
Pharmaceutical & Biotechnology Companies: The pharmaceutical & biotechnology companies segment holds a dominant 62.4% share in the lentivirus manufacturing for advanced therapies market. The pharmaceutical and biotechnology companies segment is the dominant end-user in the market by playing a crucial role in developing and commercializing gene and cell therapies. Pharmaceutical and biotech companies are increasingly investing in research and development (R&D) activities for gene and cell therapies, which are the vital applications for lentiviral vectors.
Research Institutes & Academic Institutions: On the other hand, the research institutes & academic institutions segment is the fastest-growing in the lentivirus manufacturing for advanced therapies market. Research institutes & academic institutions play a significant role in early-stage research, foundational discovery, and preclinical development of cell and gene therapies. Research institutes and academic institutions widely use lentiviruses as essential tools for the preclinical and clinical development of new therapeutic genes.
Lentivirus Manufacturing for Advanced Therapies Market Regional Insights
The North America region dominates the lentivirus manufacturing for advanced therapies market. The North American region is a major hub for innovation and the production of advanced therapies. The region is home to several dominant CDMOs and biotech companies, including Catalent, Thermo Fisher Scientific, Lonza, AGC Biologics, and Charles River Laboratories, which are continuously innovating and expanding their manufacturing capabilities. Factors such as rising preclinical development of cell and gene therapies, a supportive regulatory framework, significant investment in R&D, increasing demand for outsourced services, and the rising prevalence of chronic diseases are expected to accelerate the growth of the lentivirus manufacturing for advanced therapies market during the forecast period.
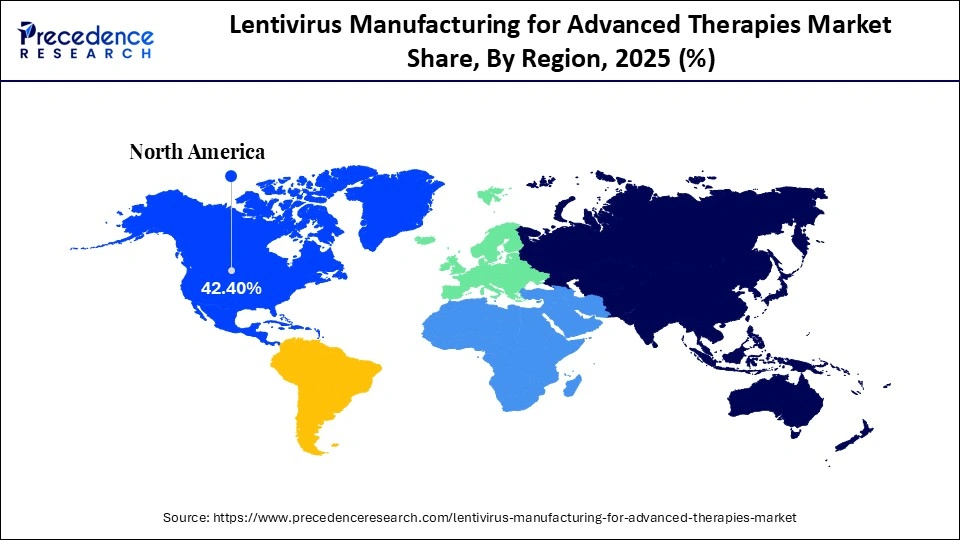
The country holds the leadership and holds a majority of the share in the North America lentivirus manufacturing for advanced therapies market, owing to its robust presence of pharmaceutical/biotechnology sectors, a well-established R&D infrastructure, a rising number of ongoing clinical trials, and the increasing burden of chronic diseases such as cancer and rare genetic disorders. The U.S. Food and Drug Administration (FDA) has provided a favorable regulatory landscape for approvals of cell & gene therapies, CAR-T therapies, cancer immunotherapies, and other therapies.
In May 2025, Kytopen Corp. and Bio-Techne Corporation announced a partnership to streamline gene delivery solutions for the development and manufacture of advanced cell therapies. The collaboration will focus on the synergies developers can achieve by utilizing the TcBuster GMP non-viral genome engineering system and the Flowfect Tx GMP cellular engineering platform. Together, these technologies offer a clear path to shorter workflows for generating more efficient genome-engineered immune cell therapies, expediting their advancement to clinical stages and subsequent manufacturing.
The Asia Pacific region is the fastest-growing market for lentivirus manufacturing for advanced therapies. The region hosts a large concentration of pharmaceutical and biotechnology companies that continue to invest heavily in cell and gene therapy pipelines. These firms are advancing programs in oncology, rare diseases, regenerative medicine, and vaccine development, which increases demand for high-quality lentiviral vectors. As clinical activity expands, manufacturers require scalable production systems, GMP-compliant facilities, and reliable supply chains to support both early research and late-stage clinical programs.
Public and private sector investments are rising across the region. Governments in countries such as China, Japan, South Korea, and Singapore are funding national initiatives focused on gene therapy development, biomanufacturing, and translational research. This includes support for vector manufacturing sites, regulatory modernization, and partnerships with global technology providers. At the same time, private investors are accelerating capital flow into cell therapy start-ups, vector design companies, and CDMO platforms.
India's Lentivirus Manufacturing for Advanced Therapies Market Analysis
India's lentivirus manufacturing for the advanced therapies market is experiencing growth. The country's growth is supported by increasing investment in cell & gene therapies, a strong presence of pharma/biotech and CDMOs, a surge in R&D Investments, and supportive regulatory frameworks. In addition, the increasing prevalence of target diseases such as cancer and rare genetic disorders in the region creates a significant demand for effective, advanced therapeutic solutions. Such a combination of factors is anticipated to fuel the expansion of the lentivirus manufacturing for advanced therapies market in the coming years.
In March 2025, Bharat Biotech International Limited (BBIL) announced the launch of India's only vertically integrated, purpose-designed Cell & Gene Therapy (CGT) Infrastructure & Viral Vector Production Facility at Genome Valley, expanding its expertise from vaccine innovation to leading-edge regenerative and personalised therapies.
The European market holds a substantial market share. The region is experiencing notable growth, owing to the significant demand for lentiviral vectors needed for an increasing pipeline of cell and gene therapies, particularly for cancer and genetic disorders. Several biopharmaceutical companies are increasingly collaborating with Contract Development and Manufacturing Organizations (CDMOs) that offer specialized expertise, advanced technologies, and established cGMP facilities for lentiviral vectors production. Additionally, the rapid technological innovations have improved the manufacturing efficiency, scalability, and cost-effectiveness of advanced therapies, boosting the region's growth during the forecast period.
In September 2024, Rentschler Biopharma SE, a leading global contract development and manufacturing organization (CDMO) for biopharmaceuticals, including advanced therapy medicinal products (ATMPs), announced the launch of an expanded service offering at its dedicated advanced therapies site in Stevenage, UK. The expanded capabilities offer a comprehensive suite of solutions in the advanced therapy landscape.
Germany Lentivirus Manufacturing for Advanced Therapies Market Analysis
Germany is poised for significant growth, attributed to an expanding pipeline of cancer-focused gene therapies, ongoing advancements in cancer immunotherapy, rising research funding, rapid technological advances in lentivirus manufacturing for advanced therapies, and a favourable government framework. Additionally, increasing outsourcing trends and growing demand for advanced gene and cell therapies to treat cancer, rare genetic disorders, and infectious diseases are expected to accelerate the market expansion in the country during the forecast period.
Key players in the Lentivirus Manufacturing for Advanced Therapies Market
- Thermo Fisher Scientific
- Oxford Biomedica
- Miltenyi Biotec
- Charles River Laboratories
- Catalent Inc.
- Fujifilm Diosynth Biotechnologies
- Cytiva
- Takara Bio Inc.
- Vigene Biosciences (part of Charles River)
- Bluebird Bio
- Cobra Biologics Limited
- Sirion Biotech GmbH
- Merck KGaA
- Sartorius AG
Recent Developments
- In June 2024, Charles River Laboratories International, Inc. and the Gates Institute at the University of Colorado Anschutz Medical Campus announced an agreement to establish a lentiviral vector contract development and manufacturing organization (CDMO). Gates Institute will leverage Charles River's premier cell and gene therapy CDMO expertise to develop Good Manufacturing Practice (GMP)-grade lentiviral vectors (LVVs) for use in novel chimeric antigen receptor (CAR) T-cell therapies for hematological cancers.(Source: https://www.nasdaq.com)
- In November 2025, AGC Biologics announced a new manufacturing agreement with the biotechnology company, marking AGC Biologics' latest advancement in the adeno-associated virus market. Under this agreement, AGC Biologics will provide Good Manufacturing Practice manufacturing for AAVantgarde's two novel candidates designed to address progressive and irreversible vision loss, where there are currently no approved therapies, such as AAVB-039 for Stargardt disease and AAVB-081 for retinitis pigmentosa (caused by Usher syndrome type 1B).(Source: https://www.businesswire.com)
Lentivirus Manufacturing for Advanced Therapies Market Segments Covered in the Report
By Application
- Gene Therapy
- Vaccines/Vaccinology
- Cancer Immunotherapy
- Others
By End-User
- Pharmaceutical & Biotechnology Companies
- Research Institutes & Academic Institutions
- CROs/CDMOs
- Others (hospitals, clinical-trial sponsors)
By Scale of Operation
- Clinical/Pilot-scale Production
- Commercial/Large-scale Production
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content