What is Pharmaceutical Contract Manufacturing and Research Services Market Size?
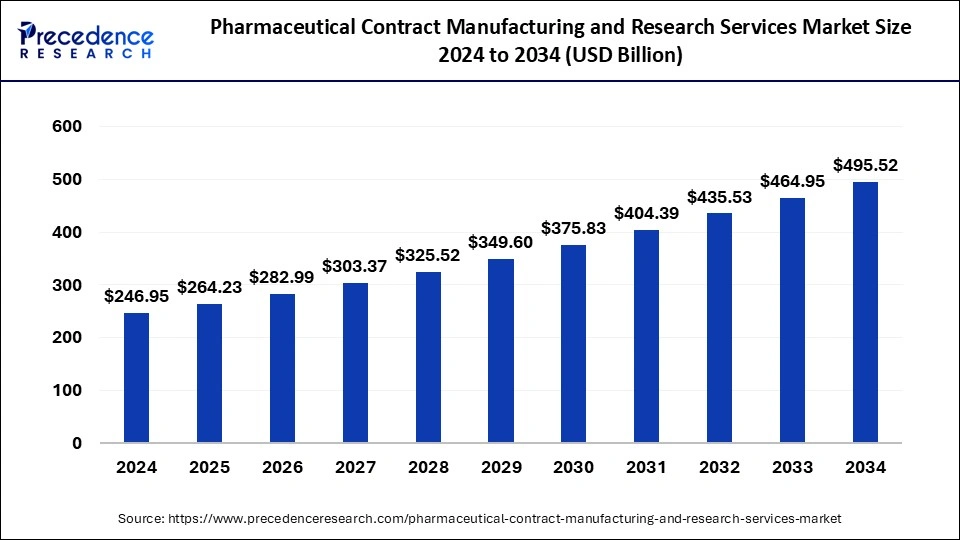
The global pharmaceutical contract manufacturing and research services market size accounted for USD 264.23 billion in 2025 and is predicted to increase from USD 282.99 billion in 2026 to approximately USD 525.32 billion by 2035, expanding at a CAGR of 7.11% from 2026 to 2035.
Market Highlights
- Asia Pacific led the global market with the highest of 41.58% market share in 2025.
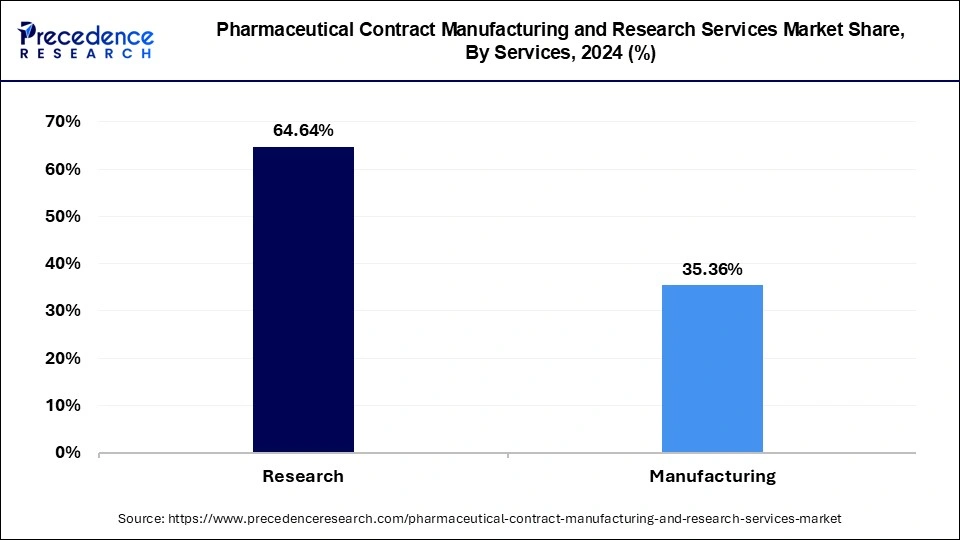
- By service, the manufacturing segment has accounted highest market share of around 64.64% in 2025.
- By service, the finished dose formulations segment is growing at a CAGR of 5.81% from 2026 to 2035.
- By manufacturing, the API/bulk drugs segment has contributed 46.81% market share in 2025.
- By research service, oncology has generated a 32.57% revenue share in 2025.
MarketOverview
Pharmaceutical contract manufacturing is a procedure in which pharmaceutical drugs are delivered on a contract basis. Moreover, drug company engages a production company to make their drugs for them, and drug companies are generating a crucial number of products that can frequently simplify their creations by joining a manufacturing company.
In a contract, manufacturing is also referred to as a company's production of goods under another company's label or trademark. The contract manufacturers give services to a variety of businesses based on their designs and the requirements of their customers. While a contract research organization is a corporation that provides support to the pharmaceutical and medical device sectors in the form of contract manufacturing and research services.
The contract research organizations are designed to help corporations develop innovative medications and pharmaceuticals in specialized markets by lowering costs. The earlier manufacturer incorporates these into its product manufacturing process. A contract manufacturing organization is a company that offers to the pharmaceutical business and offers a full range of services, from medication discovery to production.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 264.23 Billion |
| Market Size in 2026 | USD 282.99 Billion |
| Market Size by 2035 | USD 525.32 Billion |
| Growth Rate from 2026 to 2035 | CAGR of 7.11% |
| Largest Market | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Service, and End-User, and region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Increasing generics demand, rising pharmaceutical research and development investments, and contract manufacturing and research services investments in advanced manufacturing technologies are driving global pharmaceutical contract manufacturing and research services market growth. The growing demand for biological therapies, a greater emphasis on specialty medicines, expansion in the nuclear medicine sector, and advances in cell and gene therapies are all expected to provide pharmaceutical contract manufacturing and research services market growth opportunities in the coming years.
A significant solution from procurement-based outsourcing is the focus of large pharmaceutical companies on strategically engaging with a small number of preferred providers. This was one of the many business characteristics that this service industry had come to expect. The service providers with the potential to provide cost and quality advantages are ready to make significant inroads and grow rapidly during the forecast period.
Many biotech and pharmaceutical businesses are outsourcing their research development to contract research groups due to increasing technological advancements and expanding globalization. Additionally, increasing investments by major prominent vendors in various clinical and non-clinical research activities outsourced by many contract research organizations services that help in cost-effective options for development products will boost the growth of the global pharmaceutical contract manufacturing and research services market during the forecast period.
The rising need for effective medications and healthcare equipment, as well as the high cost of product development and the growing patient population, are expected to drive the global pharmaceutical contract manufacturing and research services market throughout the forecast period. In addition, the pharmaceutical contract manufacturing and research services market revenue will be driven by the strict medication approval process and expanding strategic collaboration among several prominent vendors throughout the forecast period.
A key factor driving the growth of the pharmaceutical contract manufacturing and research services market is the patent expiration of various medications. Although the downfall of patents has resulted in major income and volume losses for the branded pharmaceutical business, patent expiration allows lots of new cheaper generic alternatives to enter the pharmaceutical contract manufacturing and research services market. As generic organizations outsource their production to contract manufacturing and research services, this is a favorable indicator for the growth of the pharmaceutical contract manufacturing and research services market during the forecast period.
Another key factor driving the growth of the global pharmaceutical contract manufacturing and research services market is the lower cost of producing active pharmaceutical ingredients (API), increased focus on cost reduction, and rising pricing pressure. Additionally, one of the major factors driving the growth of the pharmaceutical contract manufacturing and research services market includes staff, technology, and infrastructure, as well as increased demand as a result of the ongoing biologic drug patent cliff.
On other hand, the pharmaceutical contract manufacturing and research services market's growth is restrained due to the stringent government regulatory frameworks and their limitations, as well as continuous improvement in the strict laws imposed by governments of emerging nations. Furthermore, due to the highly personalized character of gene and cell treatments, they can meet unmet medical requirements in the treatment of a variety of diseases or illnesses. Many pharmaceutical companies and investors have invested large resources in developing and commercializing these medicines because of their strong therapeutic potential. Thus, the above-mentioned factors are driving the growth of the market.
The increasing number of cell therapy applicants, combined with their rapid progression through the numerous stages of clinical development and their complex manufacturing process, is increased demand for facilities that provide these therapies' manufacturing services which resulting driving the growth of the market.
Segment Insights
Services Insights
The biologics segment dominated the pharmaceutical contract manufacturing and research services market in 2025. The rising demand for vaccines and biosimilars is a crucial factor driving the segment's growth in the pharmaceutical contract manufacturing and research services market during the forecast period.
Pharmaceutical Contract Manufacturing and Research Services Market Revenue, By Services 2022-2024 (USD Billion)
| By Services | 2022 | 2023 | 2024 |
| Manufacturing | 137.45 | 148.40 | 159.62 |
| Research | 77.85 | 82.61 | 87.33 |
End-User Insights
The big pharmaceutical companies segment dominated the pharmaceutical contract manufacturing and research services market in 2024. The huge share of this end-user category can contribute to the growth of the market such as big pharmaceutical companies' strong need for end-to-end services, rising pricing pressure and channel issues in their operations, and the growing need to optimize execution costs as bestselling medication patents expire.
Pharmaceutical Contract Manufacturing and Research Services Market Revenue, By End User 2022-2024 (USD Billion)
| By End User | 2022 | 2023 | 2024 |
| Big Pharma | 92.95 | 98.83 | 104.68 |
| Small Pharma |
48.19 | 51.88 | 55.65 |
| Generic Pharma |
40.43 | 43.74 | 47.15 |
| Contract Research Organizations |
33.72 | 36.55 | 39.48 |
Regional Insights
Asia Pacific Pharmaceutical Contract Manufacturing and Research Services Market Size and Growth 2026 to 2035
The Asia Pacific pharmaceutical contract manufacturing and research services market size is exhibited at USD 109.36 billion in 2025 and is projected to be worth around USD 208.01 billion by 2035, growing at a CAGR of 6.64% from 2026 to 2035
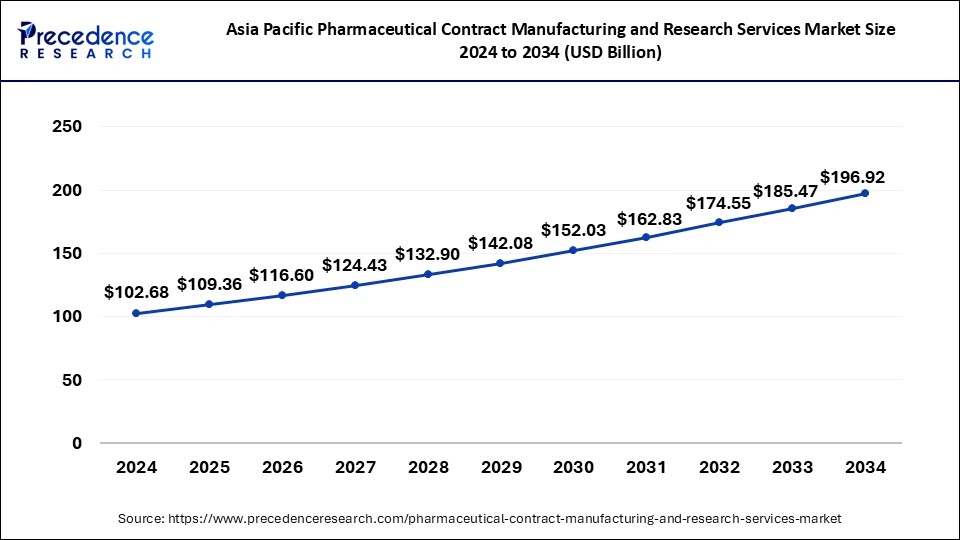
Asia Pacific dominated the pharmaceutical contract manufacturing and research services market in 2025. The growth of the region is attributed to its large base of generic drug manufacturers, growing scientific base and capability, and enhanced manufacturing facilities by major key players in the last two to three years. Additionally, Low labor and manufacturing costs have been the major factor that attracted major investors from pharma companies in developing economies such as China and India.
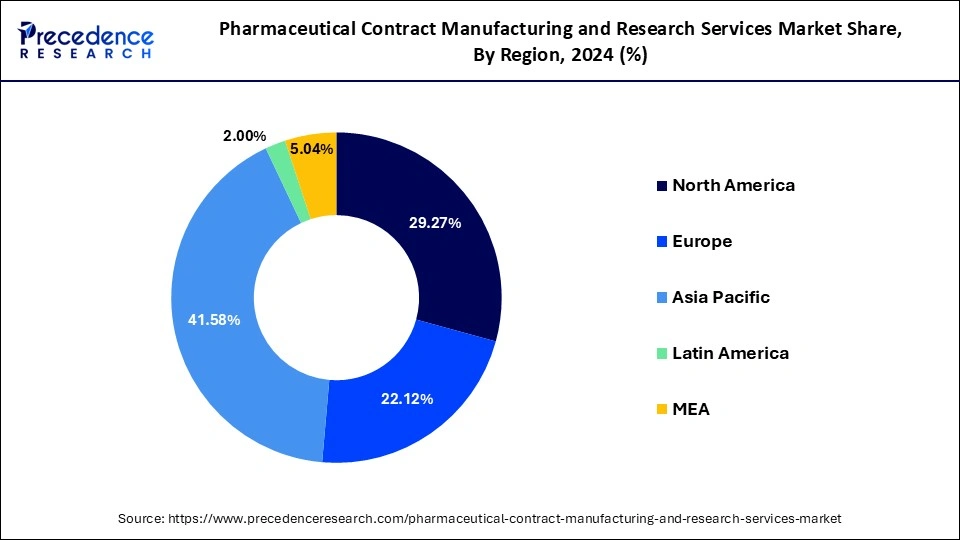
The North America is estimated to witness extensive growth over the forecast period owing to the high number of clinical trials, broad active pharmaceutical ingredients production base, modern manufacturing capabilities, presence of top pharmaceutical companies, and expansion in the generics industry are all factors that contribute to the North American region's is estimated to grow at a significant rate over the forecast period.
Europe, on the other hand, is expected to develop at the fastest rate during the forecast period. Due to a rapidly ageing population, a widening imbalance between demand and supply for pharmaceutical products, the availability of a skilled workforce, and favorable government policies in developing countries in this region.
Pharmaceutical Contract Manufacturing and Research Services Market Companies
- Thermo Fisher Scientific Inc.
- Recipharm AB
- Piramal Pharma Solutions
- Jubilant Pharmova Limited
- Curia Global, Inc.
- Cambrex Corporation
- CordenPharma International
- Samsung Biologics
- FAMAR Health Care Services
- WuXiAppTec
Recent Developments
- In February 2022, Recipharm AB (Sweden) a leading contract development and manufacturing organization declared the acquisition with Arranta Bio. The purpose of the acquisition with Arranta Bio is to offer innovative drug developers in the Biologics market scientifically distinguished contract development and manufacturing services for advanced therapy medicinal products
- In June 2020 Catalent Inc a leading provider of advanced delivery technologies, development, and manufacturing solutions for drugs, biologics, cell and gene therapies, and consumer health products, announced a partnership with Moderna, Inc. The purpose of this collaboration is to develop a large-scale, commercial fill-finish manufacturing process for Moderna's mRNA-based COVID-19 vaccine candidate.
- Apr. 2020 ICON PLC a leading provider of drug and device development and commercialization services to the pharmaceutical, biotechnology, and medical device industries, declared its agreement with Pfizer Inc. The purpose of this agreement is to provide medication and device research and commercialization services to Pfizer Inc. in April 2020.
- Jul 2021, Samsung BioLogics a leading contract development and manufacturing organization signed a strategic partnership agreement with Kineta. The agreement's purpose is to provide end-to-end CDMO service from cell line development, clinical drug substance, and drug product manufacturing services to support IND filing for KVA12.1.
Segments Covered in the Report
By Service
- Manufacturing
- API/Bulk Drugs
- Advanced Drug Delivery Formulations Packaging
- Packaging
- Finished Dose Formulations
- Solid Formulations
- Liquid Formulations
- Semi-solid Formulations
- Research
- Oncology
- Vaccines
- Inflammation & Immunology
- Cardiology
- Neuroscience
- Others
By End-User
- Big Pharma
- Small Pharma
- Generic Pharma
- Contract Research Organizations
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting