Allogeneic Cell Therapy Devices Market Will Grow at CAGR of 25.28% By 2032
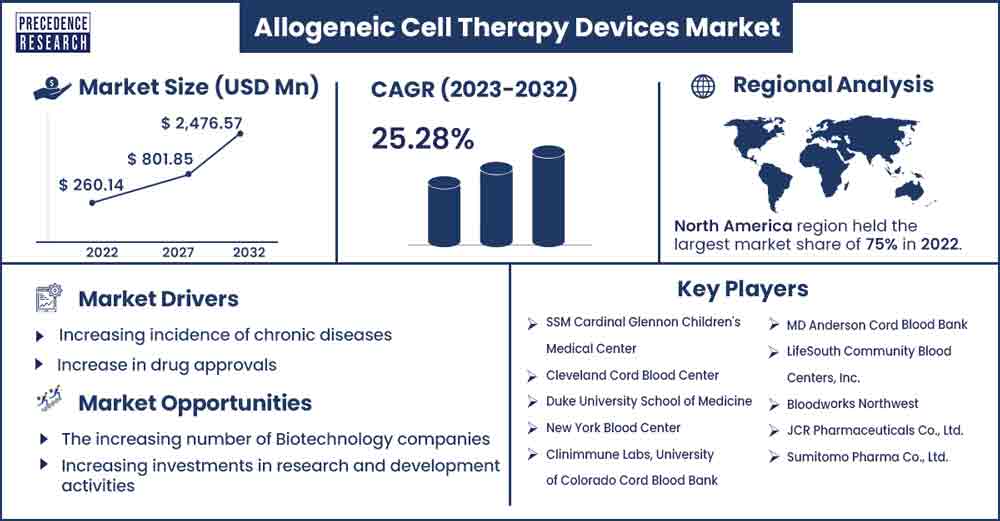
The global allogeneic cell therapy devices market size was exhibited at USD 260.14 million in 2022 and is anticipated to touch around USD 2,476.57 million by 2032, expanding at a CAGR of 25.28 % from 2023 to 2032.
Market Overview
In recent decades, cell therapies have been promising new drug products that treat or cure various diseases. These therapies are manufactured in advance and immediately available, eliminating the need for individualized production for every patient. Allogeneic cell therapy refers to a therapy using cells from a donor to be given to several patients. They rely on a single source of cells and use cells from healthy donors that can be modified before they are used to treat several patients.
Allogeneic cell therapy products are well known for generating encouraging pre-clinical and clinical results and have the high potential to serve as treatments for a wide range of chronic diseases. Also called off-the-shelf therapies, allogeneic cells are derived from a healthy donor, not the patient, and are available at the time of need. These help to respond to an illness effectively and are widely used in treating patients.
The growth of the global allogeneic cell therapy devices market is driven by several factors, including growth in healthcare infrastructure, increasing demand for personalized medicine, rise in drug discovery and development, increase in clinical trials, rising significance of the regenerative medicine field, technological advancements in biotechnology, and increasing investment in research and development activities. Additionally, the market is also impacted by the increasing cases of chronic diseases such as cancer, autoimmune disorders, Lupus, rheumatoid arthritis (RA), multiple sclerosis (MS), psoriasis, Sjögren's disease, Crohn's disease, and others. The surge in chronic diseases has spurred the demand for allogeneic cell therapy devices.
- In July 2023, Pfizer announced their investment in Caribou Biosciences, which was worth USD 25 million. This move would give the gene-editing company a vote of confidence and a boost of funding for allogeneic CAR-T cell therapy for multiple myeloma.
- In May 2023, US-based StemCures announced an investment worth USD 54 million to establish India's largest stem cell manufacturing laboratory at Hyderabad in India.
- In August 2023, Astellas Pharma Inc. and Poseida Therapeutics, Inc. announced a strategic investment to accelerate the advancement of Poseida's commitment to redefining cancer cell therapy. Astellas to invest a total of $50 million in acquiring approximately 8.8% of Poseida and in receiving a right of exclusive negotiation and first refusal for any potential partnering of P-MUC1C-ALLO1, an allogeneic CAR-T cell therapy product candidate for solid tumors.
Regional Insight
North America accounted for the larger market share during the forecast period. The growth of the region is attributed to the high adoption of allogeneic cell therapy, increasing R&D expenditure, the presence of well-established healthcare infrastructure, rising drug discovery activities, and increasing prevalence of chronic diseases such as cardiovascular, cancer, neurological disorders, diabetes, and others.
Among all countries in the region, the United States market is expected to grow at a rapid CAGR during the forecast period due to the rise in healthcare expenditure, increasing clinical trials, increase in allogeneic cell therapy applications, rising research and development investment by the government, and increasing drug discoveries and approvals. The growth of the market is also driven by the wide adoption of strategic approaches such as partnership or collaboration.
- In October 2022, Century Therapeutics collaborated with Bristol Myers Squibb to develop and commercialize iPSC-derived allogeneic cell therapies for hematologic malignancies and solid tumors. Century Therapeutics will receive USD 150M in cash ($100M upfront payment and $50M equity investment), with the potential for an additional $3B in payments plus royalties on global net product sales across multiple programs in hematologic malignancies and solid tumors. Thus, this is expected to propel the market growth in the region during the forecast period.
Allogeneic Cell Therapy Devices Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 325.77 Million |
| Projected Forecast Revenue by 2032 | USD 2,476.57 Million |
| Growth Rate from 2023 to 2032 | CAGR of 25.28% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increase in drug approvals
The increase in drug approvals by the U.S. Food and Drug Administration is anticipated to fuel the market's growth. Researchers are continuously working to discover advanced drugs to treat patients with chronic diseases. For instance, In April 2023, the U.S. FDA approved Omisirge, a substantially modified allogeneic (donor) cord blood-based cell therapy to quicken the recovery of neutrophils in the body and reduce the risk of
infection. The product is intended for use in adults and pediatric patients 12 years and above with blood cancers planned for umbilical cord blood transplantation following a myeloablative conditioning regimen (treatment such as radiation or chemotherapy). In addition, the growth of the pharmaceutical and biotechnology sector has resulted in an increasing number of drug discoveries and enabled the speedy as well as effective commercialization of therapeutics. Thereby driving the market's growth.
The rising burden of chronic diseases
The increasing incidence of chronic disorders such as cancer, autoimmune, neurological disorders, cardiovascular, diabetes, and others is expected to boost the growth of the allogeneic cell therapy market. According to the CDC, 6 in 10 Americans live with at least one chronic disease, such as heart disease, stroke, cancer, and diabetes. These chronic disorders are the leading causes of death globally. Therefore, with increasing cases of chronic diseases, the demand for effective and advanced medical treatments, such as allogeneic cell therapy, is growing, and patient outcomes are improving, which is expected to fuel the growth of the market in the coming years.
Restraints
Stringent government regulation
Stringent government regulations are projected to hamper the growth of the global allogeneic cell therapy devices market—health authorities, such as the FDA and EMA, impose these rigorous regulatory policies. In addition, the high cost is required for research, preclinical studies, clinical trials, and commercialization and is likely to limit the expansion of the global allogeneic cell therapy devices market during the forecast period.
Lack of infrastructure for allogeneic cell therapies
The need for more infrastructure for allogeneic cell therapies is likely to restrain the market's growth. The absence of sophisticated infrastructure for allogeneic cell therapies in many underdeveloped and developing countries acts as a key challenge to the growth of the allogeneic cell therapy devices market during the forecast period.
Opportunities
The increasing number of Biotechnology companies
The increase in the number of biotechnology companies is projected to offer lucrative growth opportunities for the market growth. Biotechnology companies are pursuing
allogeneic therapies, those developed from donor cells. Allogeneic cell therapies are immediately available to patients with little or no wait time. In recent years, the biotechnology industry has grown at a rapid speed. Allogeneic cell therapies in biotechnology companies are driven by the increasing focus on treating patients with chronic diseases.
Increasing investments in research and development activities
The rising investments in research and development activities are expected to propel the market's growth during the forecast period. The market has witnessed growth due to the increasing investments by governments and private and public organizations in research and development. For instance, in November 2022, the California Institute for Regenerative Medicine (CIRM) Board awarded over USD 6 million to Jianhua Yu at the Beckman Research Institute of City of Hope for developing a new approach to target hypoxia metastatic breast tumors with allogeneic off-the-shelf anti-EGFR CAR NK cells. Stem cell research is advancing rapidly, with new findings and discoveries. In addition, the increasing focus on clinical trials from autologous to allogeneic cell therapy for various chronic diseases, such as cancer, genetic disorders, autoimmune diseases, and others, is accelerating the market's growth.
Recent Developments
- In October 2022, Lonza, a global manufacturing partner to the pharma, biotech, and nutrition industries, is expanding its cell and gene therapies process and analytical development laboratories in Houston (U.S.) and Geleen (NL).
- In August 2022, RoslinCT and a contract development and manufacturing organization, Lykan Bioscience, collaborated to create advanced cell therapies.
- In January 2023, Sana Biotechnology, Inc. received approval from the FDA to initiate a first-in-human study of SC291, a CD19-targeted allogeneic CAR-T cell therapy, in patients with B-cell malignancies.
Key Market Players
- SSM Cardinal Glennon Children's Medical Center
- Cleveland Cord Blood Center
- Duke University School of Medicine
- New York Blood Center
- Clinimmune Labs, University of Colorado Cord Blood Bank
- MD Anderson Cord Blood Bank
- LifeSouth Community Blood Centers, Inc.
- Bloodworks Northwest
- JCR Pharmaceuticals Co., Ltd.
- Sumitomo Pharma Co., Ltd.
- Atara Biotherapeutics
- Mallinckrodt Pharmaceuticals
- Tego Science Inc
- Takeda Pharmaceutical Company Limited
- STEMPEUTICS RESEARCH PVT LTD
- Biosolution Co., Ltd.
- MEDIPOST Co., Ltd.
Market Segmentation
By Therapy Type
- Stem Cell Therapies
- Non-stem Cell Therapies
By Therapeutic Area
- Hematological Disorders
- Dermatological Disorders
- Others
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3369
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308