Cardiotoxicity Screening Market Revenue to Attain USD 7.59 Bn by 2033
Cardiotoxicity Screening Market Revenue and Trends 2025 to 2033
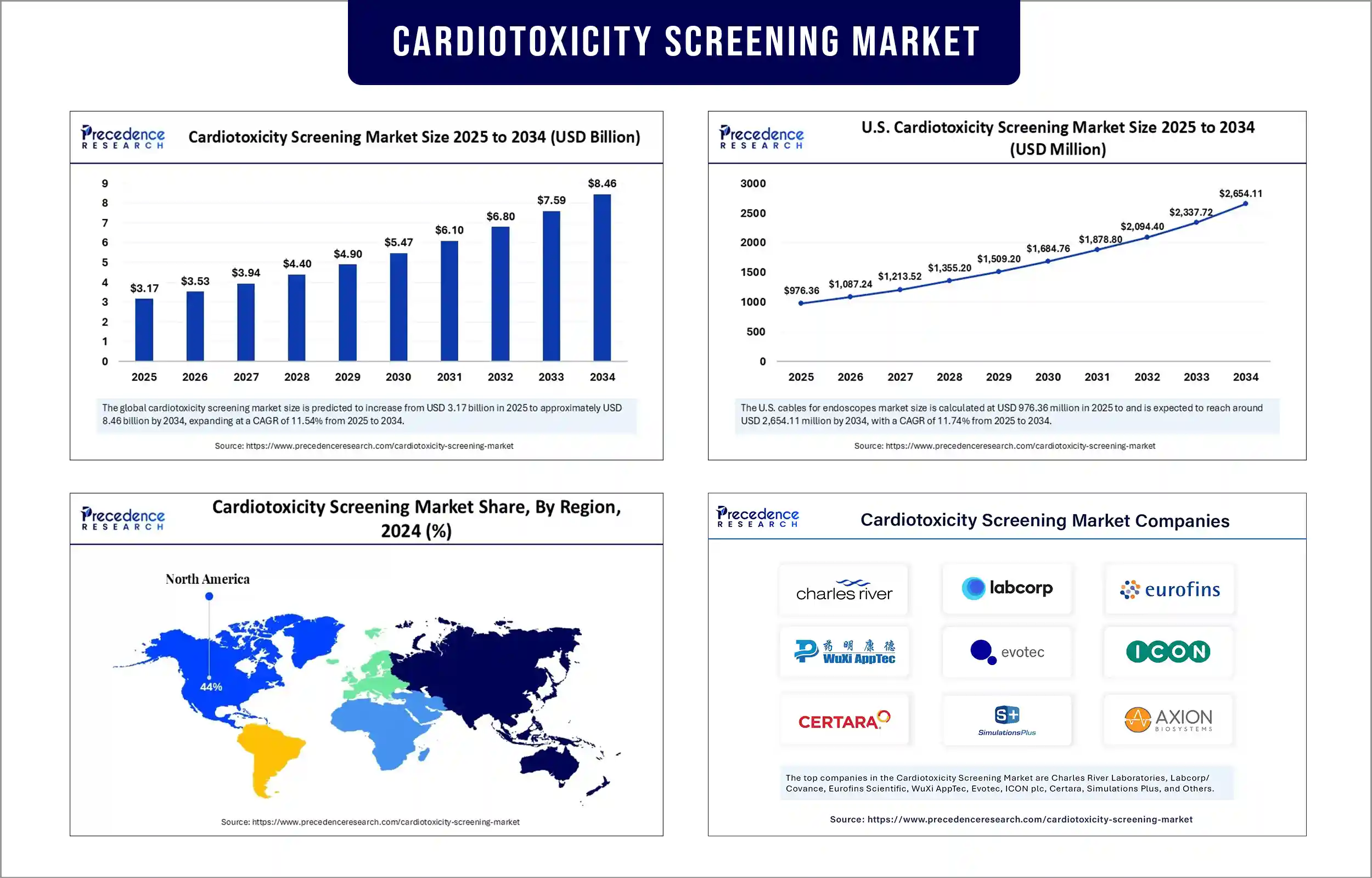
The global cardiotoxicity screening market revenue surpass USD 3.17 billion in 2025 and is predicted to attain around USD 7.59 billion by 2033, growing at a CAGR of 11.54%. This market is rising due to increasing demand for safer drug development, stringent regulatory requirements, and growing adoption of advanced in vitro and predictive testing technologies.
Key Drivers Influencing the Growth of Cardiotoxicity Screening Market
The cardiotoxicity screening market is primarily experiencing an upswing due to the increase in prevalence of cardiovascular diseases, and the need for safer drug development processes. As the pharmaceutical and biotechnology industry faces increased scrutiny to decrease later-stage failures, early and accurate assessment of cardiotoxicity must be addressed as an important aspect of drug development. Progress in in-vitro screening technologies, stem cell–based assays and high throughput screening will expand the market potential for identified solutions to meet various needs for regulators and developers.
Furthermore, monitoring policies implemented by government regulatory agencies that require drug developers to incorporate safety evaluations for broad use of therapeutics are directing developers to integrate more robust cardiotoxicity testing solutions into the process. The increase in the use of personalized medicine and value for money through predictive toxicology is placing more emphasis on drug developers to develop affordable, safe and effective measures that minimize risks to individuals and raise improved clinical outcomes.
Segment Insights
- By assay/test type, the hERG (Kv11.1) binding and patch-clamp assays as being the dominant test types based on their reliability, precision, and regulatory compliance for drug safety assessment.
- By modality/approach, the cardiotoxicity screening market, the in vitro (cell and tissue based) approaches are the best performing approach based on accuracy, scalability and cost-plus predictive drug safety interpretation.
- By product and service type, the cardiotoxicity screening market, the assay kits, reagents and ready to use cells (e.g., iPS cells - cardiomyocytes, dyes, media) are the dominating segment, with efficiency, reliability and widespread research adoption perspective.
- By end-user, the pharmaceutical and biotechnology companies (in-house screening) are the predominant user based on increased investments in R&D, regulatory requirements and increased demand for safer & faster drug development.
Regional Insights
North America Cardiotoxicity Screening Market Trends
The cardiotoxicity screening market is dominated by North America due to the presence of top pharmaceutical and biopharmaceutical companies, healthcare infrastructure, regulatory requirements from the FDA for drug safety evaluations, and high investment in research and development. North America has the benefits of access to innovative in vitro assays with ease of implementation, increased usage of predictive toxicology platforms, and new developments in drug - consideration since cardiovascular diseases are increasing and more drugs are getting approved. Partnerships between research institutions and market players folder help North America maintain their monopolistic positions in drug safety testing.
Asia-Pacific Cardiotoxicity Screening Market Trends
Asia-Pacific is noted as the fastest growing region for cardiotoxicity screening with the development of more pharmaceutical manufacturing hubs, increased government initiatives for safe drug practices, and an increase in clinical research. Countries such as China, India, and Japan are now at the forefront of global research initiatives and advances in drug research that can use lower cost research facilities as well as increased healthcare investment.
The focus on other new technologies such as in vitro cell based assays, stem cell technologies and associated regulatory changes as an improved verification of drug safety evaluations during early-stage assessments have also contributed to the increase in usage, and investment for cardiotoxicity. The continued growth and rapid expansion along with the right regulatory reforms now place them into key position as an important contributor to the global landscape for cardiotoxicity screening.
Cardiotoxicity Screening Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 3.17 Billion |
| Market Revenue by 2033 | USD 7.59 Billion |
| CAGR from 2025 to 2033 | 11.54% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Developments
- On August 14, 2025, Patanjali introduced a novel herbo-mineral formulation called Cardiogrit Gold, shown in a recent study to mitigate cardiotoxicity caused by the chemotherapy drug doxorubicin. This highlights a unique convergence of Ayurvedic medicine and modern cardiotoxicity research.
(Source: https://timesofindia.indiatimes.com)
Cardiotoxicity Screening Market Key Players
- Charles River Laboratories
- Labcorp/Covance
- Eurofins Scientific
- WuXi AppTec
- Evotec
- ICON plc
- Certara
- Simulations Plus
- Axion BioSystems
- Multi-Channel Systems (MCS)
- Nanion Technologies
- Sophion Bioscience
- Molecular Devices/Danaher group
- Fujifilm-CDI (Cellular Dynamics International)
- Ncardia (formerly Axiogenesis)
- Axol Bioscience
- Thermo Fisher Scientific
- PerkinElmer
- Charles River/HESI/CiPA collaborators
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6624
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344