Next-Gen Delivery Systems for Genetic Drug Market Size and Forecast 2025 to 2034
The next-gen delivery systems for genetic drug market is accelerating with breakthroughs in delivery technologies such as lipid nanoparticles, viral vectors, and exosomes. These innovations are improving the precision, safety, and efficiency of gene and RNA-based therapies across various therapeutic areas. This market is growing due to its ability to enhance targeted delivery, stability, and safety of genetic therapies like mRNA, siRNA, and CRISPR.
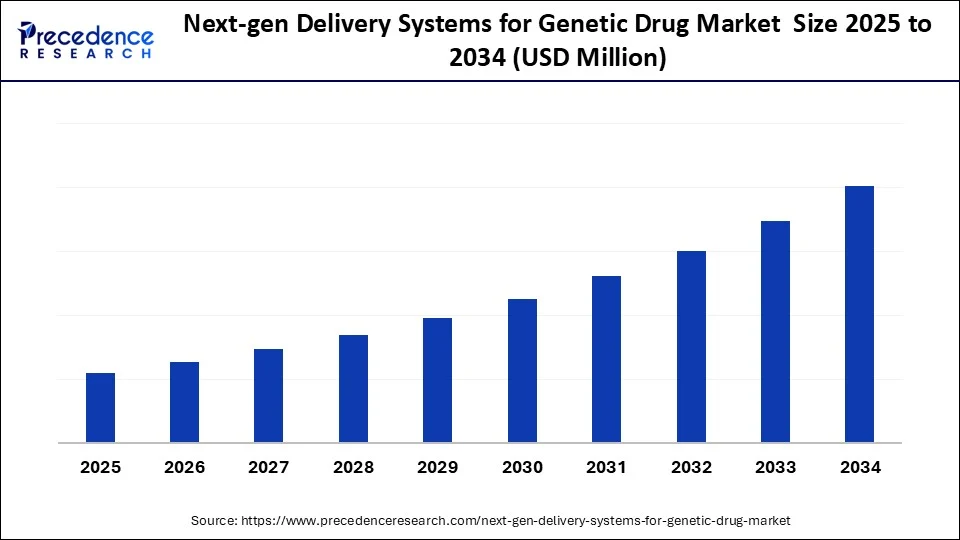
Next-Gen Delivery Systems for Genetic Drug Market Key Takeaways
- North America dominated the next-gen delivery systems for genetic drug market in 2024.
- Asia Pacific is expected to grow at a notable CAGR during the forecast period.
- By delivery system type, the lipid-based delivery segment held the largest share in 2024.
- By delivery system type, the peptide-based delivery systems segment is observed to grow at the fastest CAGR during the forecast period.
- By genetic drug type, the gene therapy (DNA-based) segment captured the biggest market share in 2024.
- By genetic drug type, RNA-based therapeutics are expected to grow at the fastest CAGR during the forecast period.
- By target organ/tissue, the liver segment contributed the highest market share in 2024.
- By target organ/tissue, the tumor/malignant tissue segment is emerging as the fastest growing.
- By administration route, the intravenous (IV) segment generated the major market share in 2024.
- By administration route, the oral segment is expected to grow at the fastest CAGR during the forecast period.
- By application, the oncology segment held the largest market share in 2024.
- By application, the neurology segment is expected to grow at the fastest CAGR during the forecast period.
- By end user, the hospitals & specialized clinics segment accounted for the significant market share 2024.
- By end user, the contract research organizations segment is expected to grow at the fastest rate during the fastest period.
How is AI Accelerating the Design and Optimization of Delivery Systems for Genetic Drugs?
Artificial intelligence is accelerating the design and optimization of delivery systems for genetic drugs like mRNA and CRISPR therapies by enabling rapid data analysis, material screening, and predictive modeling due to the growing demand for complex therapies like mRNA, siRNA, and gene editing tools that can be delivered safely, effectively, and precisely. Large-scale datasets can be analyzed by machine learning algorithms to find the best lipid or polymer formulations to forecast the behavior of nanoparticles in vivo and model drug carrier interactions prior to laboratory testing. This increases the safety rates of gene delivery platforms by decreasing trial-and-error testing, cutting down on development timeframes, and improving targeting precision.
Market Overview
The next-gen delivery systems for genetic drugs market refers to advanced technologies and platforms designed to deliver genetic therapeutics, including gene therapy, gene editing, RNA-based drugs (siRNA, mRNA, ASOs), and oligonucleotide-based therapeutics, effectively and safely into target cells or tissues. These delivery systems aim to overcome limitations such as degradation, off-target effects, immunogenicity, and low cellular uptake associated with conventional methods. Next-gen platforms include viral and non-viral vectors, lipid nanoparticles, exosomes, polymer-based carriers, and engineered delivery systems tailored for specific tissues or genetic payloads.
What is Driving the Overall Growth of the Next-Generation Delivery Systems in the Genetic Drug Market?
The next-gen delivery systems for genetic drug market space are growing rapidly due to the growing need for precise, effective and safe delivery of complicated treatments like gene editing tools mRNA and siRNA the limitations of traditional delivery methods such as immune responses off target effects and low stability have led to innovations in lipid nanoparticles viral vectors exosomes and polymeric carriers. Adoption of advanced delivery technologies across therapeutic areas is being further accelerated by the exploration of green and cell therapy development, regulatory approvals, and precision medicine advancements.
Next-Gen Delivery Systems for Genetic Drug Market Growth Factors
- Rising Use of Genetic Therapies: The growing use of mRNA, CRISPR, and siRNA therapies is boosting demand for safe and efficient delivery systems.
- Advancements in Nanotechnology & LNPs: Nanocarriers like lipid nanoparticles enable targeted, stable, and low-toxicity delivery of genetic drugs.
- AI-Powered Design & Optimization: AI is helping design smarter, faster, and more targeted delivery systems by predicting how drugs interact in the body.
- Growth in Personalized & Rare Disease Therapies: Customized delivery systems are needed for rare and personalized genetic treatments, driving innovation.
- Increased R&D and Funding: Strong investment and collaboration accelerate the development and adoption of next-gen delivery technologies.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Delivery System Type, Genetic Drug Type, Target Organ/Tissue, Administration Route, Application, End-User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising Demand for Genetic Therapies
The growing approval and development of gene-based drugs, including mRNA vaccines and CRISPR-based treatments, are creating sustained demand for efficient and precise delivery systems. Carriers that can transport genetic material to cells and prevent it from degrading are necessary for these treatments. Their application is growing due to their dual use in several therapeutic areas such as infectious diseases, rare diseases, and oncology.
The need for next-generation delivery platforms is further fueled by the more than 1000 clinical trials in the global gene therapy pipelines. With mRNA technology setting new standards for speed and efficacy, public and private sector investments in genetic therapies have also increased significantly since COVID-19. Delivery platforms are becoming essential for the success of treatments as personalized and regenerative medicine continues to grow.
Limitations of Traditional Delivery Methods
Immunogenicity scalability problems and poor targeting efficiency are problems with traditional techniques like viral vectors or simple lipid carriers. Researchers and biotech companies have shifted toward novel non-viral modular systems as a result of these constraints. In addition to enabling organ-specific targeting and reducing and reducing side effects, next-generation systems enhance payload protection.
Complex genetic medications' long-term success in both clinical and commercial settings depends on this evolution. Delivery reproducibility and safety are crucial as regulatory scrutiny grows. There is a need for safer, more adaptable, and reusable delivery methods because conventional viral-based systems are also linked to high production costs and the possibility of insertional mutagenesis.
Restraints
High Development and Manufacturing Costs
High R&D investment, specialized labor, and sophisticated infrastructure are needed to develop next-generation delivery systems, particularly nanocarriers, exosomes, and viral vectors. These technologies come with hefty upfront costs due to their intricate design testing and validation procedures. Numerous delivery systems, such as lipid nanoparticles or dendrimers, necessitate accurate, repeatable formulation techniques and specialized equipment, making large-scale manufacturing challenging. Commercialization timelines are delayed, and smaller biotechs are unable to participate due to the high capital expenditure.
Limited Stability and Storage Challenges
Temperature, light, and pH all have a significant impact on genetic payloads such as mRNA and CRISPR components. It's still changing to guarantee long-term stability even with sophisticated delivery systems. Especially in low-resource environments, cold chain logistics, particularly ultra-low storage for mRNA drugs, cause distribution bottlenecks. Instability can limit the widespread use of this treatment around the world by reducing drug efficacy, increasing production costs, and causing waste.
Opportunities
Rising Demand for Personalized Medicine and Precision Therapies
Next-generation delivery systems that are customized to each patient's unique genetic profile have enormous potential because of the growing trend toward personalized medicine. Using biomarkers unique to each patient, these systems allow for the precise delivery of therapeutic payloads to tissues or cells. The potential for creating adaptable carriers that match disease phenotype, genetic mutations, or treatment history is increasing as sequencing costs come down and data-driven healthcare grows. This customization can increase patient compliance, decrease adverse reactions, and improve medication efficacy.
Growing Interest in Nonviral Delivery Alternatives
Due to worries about the safety of viral vectors, their high cost, and the complexity of their manufacturing, non-viral delivery systems such as lipid nanoparticles, exosomes, and polymer-based carriers are becoming more popular. Benefits of these substitutes include improved scalability, reduced immunogenicity, and compatibility with various payloads. An important growth path is created as biotech companies and research labs spend money developing non-viral systems with improved targeting and controlled release capabilities, particularly for systemic and chronic illnesses.
Delivery System Type Insights
Lipid-based delivery systems dominated the next-gen delivery systems for genetic drug market due to due to their high biocompatibility, ability to encapsulate and protect fragile genetic material (like mRNA and siRNA), and successful use in approved products, such as COVID-19 mRNA vaccines. Their efficiency in cellular uptake and established regulatory pathway also made them the go-to choice for many gene therapy applications. In addition, lipid nanoparticles (LNPs) have demonstrated scalability in manufacturing, making them commercially viable. Many clinical-stage products are based on this delivery system, reinforcing its dominance in the segment.
Peptide-based delivery systems are rapidly gaining popularity because they provide improved intracellular delivery of genetic materials with low toxicity and precise targeting. Preclinical trials and research on neurological and cancer disorders are increasing as a result of their modular design, which enables customization for disease targets. To improve targeted drug release, peptides can also be engineered to react to biological stimuli. Their quick market traction can be attributed to their capacity to get past biological barriers and lessen off-target effects.
Genetic Drug Type Insights
Gene therapy (DNA-based) dominated the next-gen delivery systems for genetic drug market as it holds the capacity to replace or fix damaged genes to provide long-lasting therapeutic effects. To treat rare diseases, DNA-based therapies, particularly those that use viral vectors like AAVs, have a long history in clinical practice and have received multiple high-profile regulatory approvals. Therapeutic genes are perfect for chronic and monogenic disorders because they express themselves over an extended period of time. Their leadership has also been sustained by ongoing R&D and investments in the treatment of hereditary diseases.
RNA-based therapeutics segment is growing fastest in the next-gen delivery systems for genetic drug market driven by the global success of mRNA vaccines, increased investment in siRNA and antisense technologies, and their flexible design. These therapies are ideal for transient gene expression and are now being explored for cancer, infectious diseases, and genetic disorders. RNA drugs offer a faster development timeline compared to DNA-based approaches, and their ability to modulate gene expression without altering the genome adds to their safety profile. Pharma companies are rapidly expanding RNA-based pipelines with diverse delivery options.
Target Organ/Tissue Insights
Why are Liver-Dominating the Next-Gen Delivery Systems for Genetic Drug Market?
Liver segment dominated due to their vital role in metabolism and innate propensity to absorb nanoparticles, which makes it a perfect target for genetic medications that are administered systemically. Additionally, the liver is the site of origin for many genetic and metabolic disorders, and current delivery methods have demonstrated high success rates in efficiently targeting hepatic cells.
The special sinusoidal shape of the liver promotes the accumulation of nanoparticles, which raises the effectiveness of gene transfer. Hemophilia and hypercholesterolemia clinical trial outcomes have solidified Livers market leadership.
Tumor/malignant tissue segment is growing rapidly due to the urgent need for precision treatments in oncology. Genetic drugs that can home in on cancer cells without affecting healthy tissue are gaining traction, supported by growth in oncolytic viral therapies and tumor-specific gene editing approaches. The demand for personalized medicine in cancer care is further accelerating innovation in tumor-targeted delivery. Recent developments in tumor-penetrating peptides and pH-sensitive carriers are enabling more effective and localized drug release.
Administration Route Insights
Intravenous (IV) dominated the next-gen delivery systems for genetic drug market as it provides the most efficient and regulated way to introduce genetic medications into the bloodstream, guaranteeing the best possible bioavailability and systemic reach. It is the method of choice for the majority of clinical trials involving gene therapy and is particularly important for diseases that need extensive tissue access. IV administration encourages quick action and reduces the need for repeated dosages. For genetic medications, IV-based administration is the most clinically accessible option because hospitals are prepared to handle it.
Oral segments are rapidly expanding due to increasing demand for non-invasive, patient-friendly alternatives. Advances in nanocarriers and pH-responsive formulations are overcoming traditional stability issues, enabling successful delivery of RNA and DNA therapies through the gastrointestinal tract. The ease of self-administration can significantly improve patient compliance. R&D efforts are focused on lipid and polymer-based capsules that protect nucleic acids from enzymatic degradation during digestion.
Application Insights
Oncology segment dominated the market because of the rising need for individualized and focused cancer treatments. With the help of robust clinical trial activity and investments in cancer gene delivery platforms, gene-based therapies, such as CAR-T and tumor-specific gene editing are transforming the treatment of cancer. Innovation in this area is being driven by the prevalence of cancer worldwide and the unmet need for curative treatments. Additionally, innovative cancer medications have benefited from favorable regulatory pathways that have accelerated commercialization.
Neurology segment is growing rapidly, motivated by the pressing need for efficient therapies for neurodegenerative conditions such as Huntington's disease, ALS, and Parkinson's. Gene-based treatments for neurological disorders that were previously incurable are becoming possible thanks to the creation of delivery systems that can pass through the blood–brain barrier. Developments in nanocarriers and intrathecal delivery facilitate the delivery to certain brain regions. The market potential is also being enhanced by the growing aging population and the rising incidence of CNS disorders.
End User Insights
Hospitals & specialized clinics dominated the market due to their proficiency in delivering cutting-edge genetic therapies, regulatory compliance, and infrastructure. These facilities are essential for overseeing intricate procedures, keeping an eye on patients, and managing any unfavorable gene therapy-related events. Such clinical settings provide the sterile handling of cold-chain storage and specialized staff that gene therapy frequently requires. Additionally, the majority of clinical trials for gene therapy are conducted primarily in hospitals.
Contract research organizations (CROs) are the fastest-growing end users as pharmaceutical and biotech firms increasingly outsource research, clinical trials, and regulatory services to speed up development and reduce costs. Their specialized expertise in genetic drug testing and scalable operations is driving their rise. CROs offer tailored gene delivery platform evaluations and regulatory assistance, making them indispensable in the product development lifecycle. Partnerships with biotech startups are further expanding their influence in this domain.
Regional Insights
How is North America Dominating the Next-Gen Delivery Systems for Genetic Drug Market?
North America dominated due to its mature biotechnology ecosystem, high R&D funding, and favorable regulatory landscape. The presence of top-tier healthcare institutions, a high volume of gene therapy clinical trials, and early adoption of innovative drug delivery platforms have driven sustained growth in the region. The region also benefits from a strong collaboration network between academic researchers and biotech firms, ensuring a continuous pipeline of next-gen therapies. Government initiatives, tax incentives for R&D, and a large patient pool for rare disease trials further enhance its market position.
Asia Pacific Next-Gen Delivery Systems for Genetic Drug Market Trends
Asia Pacific is the fastest-growing region, caused by a rapidly growing population with genetic disorders, rising public and private investment in advanced therapeutics, and growing healthcare awareness. Local pharmaceutical firms are concentrating more on gene therapy research, and authorities are opening new treatment approaches. Public-private partnerships and academic collaborations to improve clinical capabilities are also growing in the region. Adoption of next-generation delivery systems is being accelerated by advancements in healthcare infrastructure and an increase in clinical trial activity.
Next-Gen Delivery Systems for Genetic Drug Market Companies

- Moderna, Inc.
- BioNTech SE
- Alnylam Pharmaceuticals
- Ionis Pharmaceuticals
- Intellia Therapeutics
- CRISPR Therapeutics
- Editas Medicine
- Beam Therapeutics
- Precision NanoSystems
- Arcturus Therapeutics
- GenEdit
- Sangamo Therapeutics
- REGENXBIO
- Voyager Therapeutics
- LogicBio Therapeutics
- Evonik Industries AG (Delivery Tech Division)
- Catalent, Inc.
- Eterna Therapeutics
- Sirnaomics
- Nitto Avecia Inc.
Recent Developments
- On 15 May 2025, IGI announced the treatment of Baby KJ with an on-demand CRISPR-based therapy developed in just six months, demonstrating a new model for rapid personalized gene editing.(Source: https://innovativegenomics.org)
- On 8 July 2025, CZI and IGI launched a new center for pediatric CRISPR cures aimed at standardizing scalable personalized gene editing therapies for severe pediatric disorders.(Source: https://innovativegenomics.org)
- On 1 July 2025, Evon unveiled progress in engineering exosome-based delivery systems designed for systemic and CNS delivery of RNA gene editing tools and proteins. (Source: https://www.pharmasalmanac.com)
- On 17 March 2025, Prime Medicine introduced an AATD gene correction program utilizing liver-targeted LNP technology showcased during a major LNP summit.(Source: https://www.precigenome.com)
Segments Covered in the Report
By Delivery System Type
- Lipid-Based Delivery Systems
- Lipid Nanoparticles (LNPs)
- Liposomes
- Solid Lipid Nanoparticles (SLNs)
- Polymer-Based Delivery Systems
- Natural Polymers (e.g., chitosan, gelatin)
- Synthetic Polymers (e.g., PLGA, PEGylated systems)
- Viral Vectors
- Adeno-Associated Virus (AAV)
- Lentivirus
- Retrovirus
- Others
- Peptide-Based Delivery System
- Exosome-Based Delivery Systems
- Inorganic Nanoparticles
- Gold Nanoparticles
- Silica Nanoparticles
- Hybrid & Emerging Systems
- Cell-Penetrating Peptides (CPPs)
- DNA Origami
- Bacterial Vectors
By Genetic Drug Type
- Gene Therapy (DNA-based)
- RNA-based Therapeutics
- mRNA Therapeutics
- siRNA
- miRNA
- Antisense Oligonucleotides (ASOs)
- Gene Editing Tools
- CRISPR/Cas9
- ZFNs
- TALENs
- Oligonucleotide Drugs
- Aptamers
By Target Organ/Tissue
- Liver
- Muscle
- CNS (Central Nervous System)
- Eye
- Lungs
- Tumor/Malignant Tissue
- Others (e.g., skin, cardiovascular)
By Administration Route
- Intravenous (IV)
- Intramuscular (IM)
- Subcutaneous (SC)
- Inhalation
- Intrathecal
- Topical
- Oral
By Application
- Rare Genetic Disorders
- Oncology
- Neurology
- Infectious Diseases
- Metabolic Disorders
- Cardiovascular Diseases
- Others
By End-User
- Biopharmaceutical Companies
- Academic & Research Institutes
- Contract Research Organizations (CROs)
- Hospitals & Specialized Clinics
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting