Bacterial Endotoxin Testing Market Revenue to Attain USD 2.77 Bn by 2033
Bacterial Endotoxin Testing Market Revenue and Trends 2025 to 2033
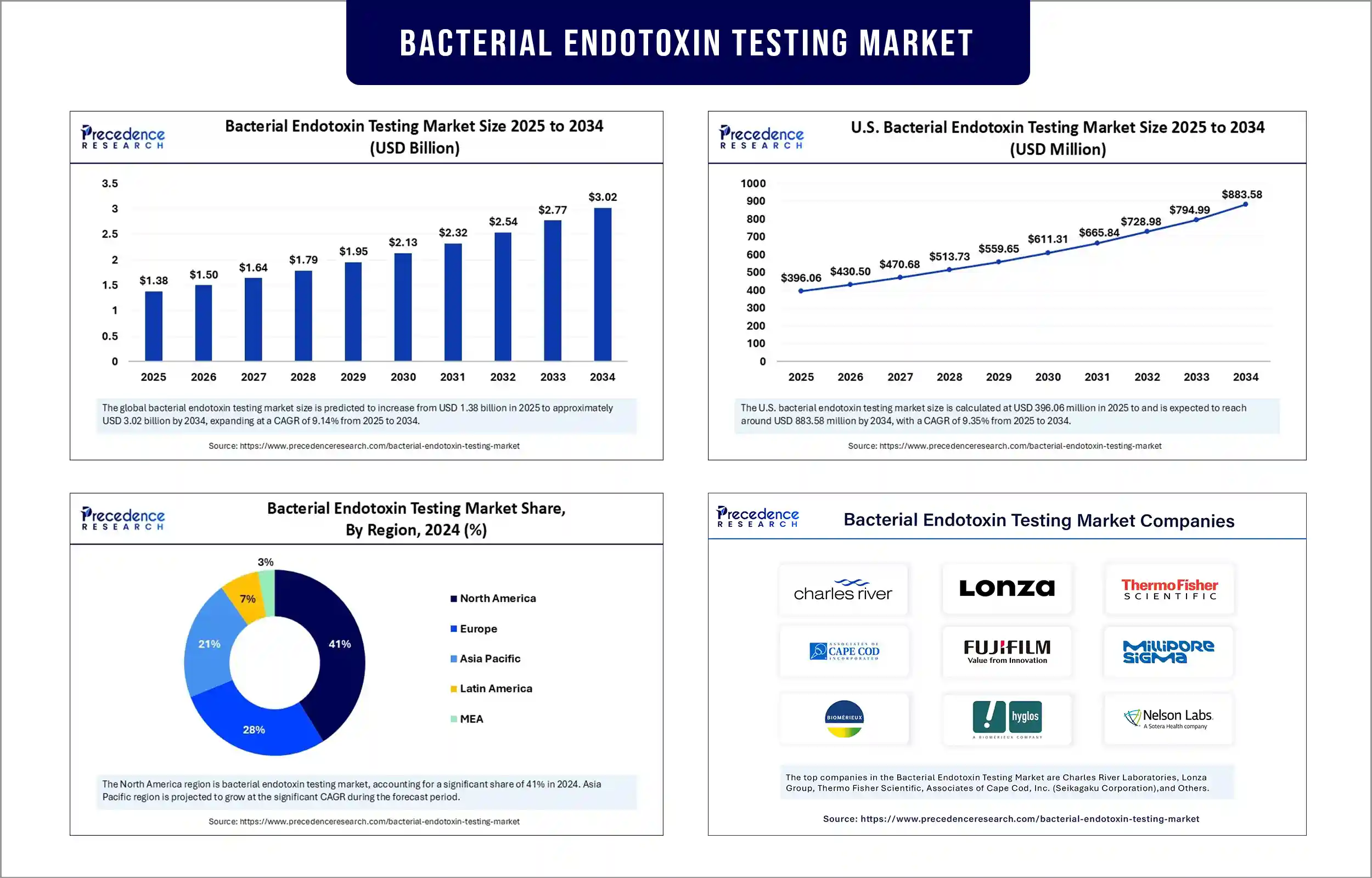
The global bacterial endotoxin testing market revenue was valued at USD 1.38 billion in 2025 and is expected to attain around USD 2.77 billion by 2033, growing at a CAGR of 9.14% during forecast period. This growth of the market is attributed to increasing production of pharmaceuticals and biologics, stringent regulatory requirements, and advancements in testing technologies.
What are the Major Factors Driving the Growth of the Bacterial Endotoxin Testing Market?
The global market for bacterial endotoxin testing is experiencing rapid growth, driven by escalating regulatory demands for safety in pharmaceuticals, biologics, and medical devices. The growing prevalence of chronic diseases and the increasing need for injectables and implantable medical devices are further intensifying the requirement for precise endotoxin detection. Moreover, technological advancements in testing systems, such as recombinant factor C assays, offer enhanced efficiency and align with animal-free testing practices. The market is also driven by increased biopharmaceuticals and vaccines production. The rising trend of outsourcing quality control testing to specialized contract research organizations further contributes to the service demand. There is a heightened awareness of product safety, reinforcing the significance of endotoxin contamination testing as a vital element of quality assurance and control.
Segment Insights
- By product type, the reagents & kits segment led the market in 2024 due to their consistent and accurate results, ease-of-use, and use across many pharmaceutical applications. They are essential in conducting reliable, rapid, and standardized tests across pharmaceutical, biotechnology, and medical device industries.
- By test type, the gel clot test segment dominated the market with the largest share in 2024. The dominance of gel clot test stems from its reliability, cost-effectiveness, and high acceptance from regulators.
- By application, the final product release testing segment led the bacterial endotoxin testing market in 2024 due to stringent regulatory requirements mandating endotoxin testing before product release. The increased production of injectables, vaccines, and implantable medical devices further augmented the segment.
- By sample type, the biologics segment dominated the market in 2024 due to the increased production of monoclonal antibodies and rigorous safety compliance requirements. Since these products are highly sensitive to contamination, there is a high demand for endotoxin testing to ensure patient safety.
- By end-user, the biopharmaceutical companies segment contributed the largest market share in 2024 as a result of their increased biologics production, which require bacterial endotoxin testing to ensure their safety and efficacy.
Regional Insights
North America sustained dominance in the bacterial endotoxin testing market by capturing the largest share in 2024. This is mainly due to its well-established biopharmaceutical and pharmaceutical manufacturing industries, stringent FDA regulations mandating high product safety standards, and a significant concentration of biopharmaceutical companies on product safety. The rising research and development funding from North American companies, coupled with their rapid adoption of new technologies like recombinant factor C assays, further bolstered regional market growth. The rising production of vaccines, biologics, and injectable and the development of automated testing solutions position North America as a leader in the market.
Asia Pacific is expected to experience the fastest growth in the market during the forecast period, driven by substantial growth in pharmaceutical and biopharmaceutical production across countries like China, India, and Japan. Rising government investments in drug manufacturing and stringent regulations regarding safety are influencing the demand for testing technologies and standards, including bacteriophage and French pharmacopeia. The region's robust production capabilities at lower costs and increasing foreign investments in life sciences will continue to drive market growth. Moreover, the rising demand for vaccines and substantial growth in clinical trials support regional market growth.
Bacterial Endotoxin Testing Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 1.38 Billion |
| Market Revenue by 2033 | USD 2.77 Billion |
| CAGR from 2025 to 2033 | 9.14% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Developments
- In January 2024, Charles River Laboratories launched the Endosafe® Trillium™ rCR cartridge, combining its Endosafe® cartridge technology with recombinant cascade reagent (rCR). This animal-free bacterial endotoxin testing solution improves efficiency, shortens manufacturing timelines, and supports sustainability goals aligned with the 4Rs, Replacement, Reduction, Refinement, and Responsibility. (Source: https://ir.criver.com)
Bacterial Endotoxin Testing Market Key Players
- Charles River Laboratories
- Lonza Group
- Thermo Fisher Scientific
- Associates of Cape Cod, Inc. (Seikagaku Corporation)
- FUJIFILM Wako Pure Chemical Corporation
- Merck KGaA (MilliporeSigma)
- bioMérieux SA
- Hyglos GmbH (A bioMérieux Company)
- Nelson Laboratories (Sotera Health)
- SGS S.A.
- WuXi AppTec
- Microcoat Biotechnologie GmbH
- Ellab A/S
- Eurofins Scientific
- BioSpectra Inc.
- BioDuro-Sundia
- PPD (part of Thermo Fisher)
- Biomerieux Industry
- Associates of Cape Cod Europe GmbH
- Microbiologics, Inc.
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6572
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344