Clinical Trials Software Industry Growth Driven by Rising Demand
Clinical Trials Matching Software Market Revenue and Trends
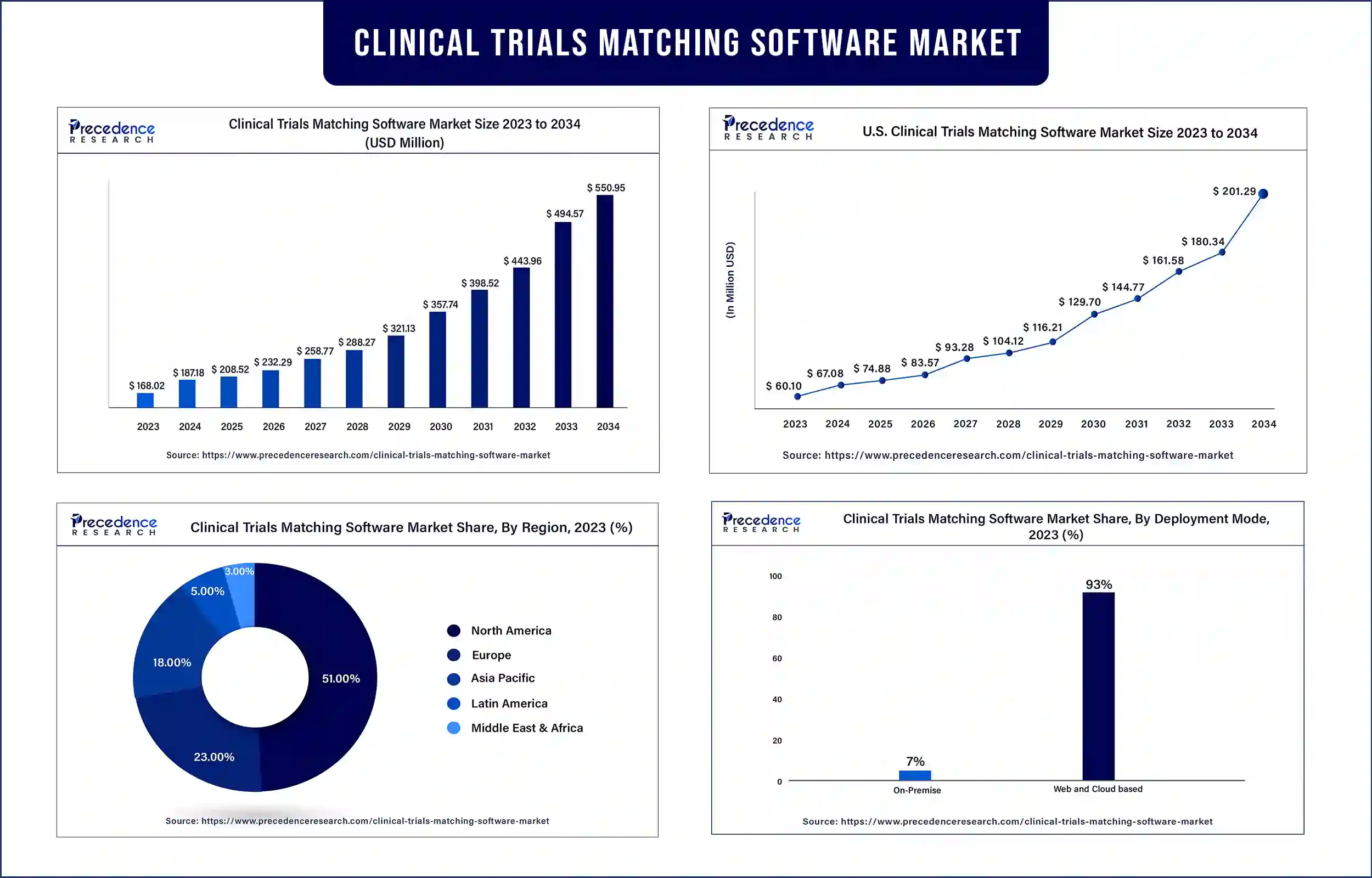
The global clinical trials matching software market revenue was valued at USD 168.02 billion in 2023 and is poised to grow from USD 187.18 billion in 2024 to USD 550.95 billion by 2034, at a CAGR of 11.4% during the forecast period 2024 – 2034. The rising need for clinical trials is expected to drive the growth of the clinical trials matching software market.
Market Overview
Manufacturers of medical devices regularly conduct clinical trials matching software market, clinical research organizations, biotechnology companies, and pharmaceutical firms to evaluate the effectiveness and security of fresh treatments, tests, and medications. The increasing prevalence of chronic diseases such as diabetes, cardiovascular diseases, and more, as well as the growing digitization of research globally, are attributed to the growth of the market.
In addition, increasing the number of clinical trial activities, increasing spending in the biopharmaceutical and pharmaceutical industries, and increasing savings by substantially investing in research and development activities for long-term returns and nurturing collaborative research are further anticipated to drive the growth of the clinical trials matching software market during the forecast period.
Clinical Trials Matching Software Market Trends
- The rising growth in technological advancements and clinical trial matching software have contributed to propelling market growth.
- The rising presence of artificial intelligence (AI) and machine learning (ML) technologies fuels the growth of clinical trial matching software.
- The increasing consumer preferences towards personalized healthcare are driving the growth of the market.
Improving Data Collection and Accuracy to Fuel Market Growth
The major importance of clinical trials is ensuring data quality and accuracy. Incomplete and inaccurate data may compromise the reliability and validity of clinical trials management systems. To detect missing data and incorporate robust data validation, the clinical trials management system is automated. This accuracy resolution can aid in enhancing reliability and data accuracy. Furthermore, the clinical trial management system permits a comprehensive review of data changes, ensuring data integrity and audit trials. Thus, these are the key factors expected to enhance the growth of the clinical trial management system market during the forecast period.
However, some factors may restrain the market growth, such as maintenance and high-cost implementation. Implementing the clinical trial management system suffers from maintenance expenditure and substantial costs, creating a major challenge for small and mid-sized companies. This financial crisis restrains their ability to optimize and utilize the clinical trial management system and creates obstacles to their participation in clinical research and trial management. Thus, these factors may restrain the growth of the clinical trial management system market.
Clinical Trials Matching Software Market Highlights
| Report Attribute | Key Statistics |
| Market Revenue in 2024 | USD 187.18 Billion |
| Market Revenue by 2034 | USD 550.95 Billion |
| Market CAGR | 11.4% from 2024 to 2034 |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2034 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Clinical Trials Matching Software Market Top Companies
- Ofni Systems
- Microsoft Corporation
- Bsi Business Systems Integration AG
- Clario
- Aris Global
- SSS International Clinical Research
- IBM Clinical Development
- Antidote Technologies, Inc.
- Advarra
- Belong. Life
Recent Innovation in Clinical Trials Matching Software by Belong.Life
- In September 2023, a leading global provider of high-engagement patient care and communication platforms, Belong. Life launched Tara, a SaaS-based conversational AI cancer clinical trial matching platform for contract research organizations (CROs) and health providers. The solution is designed to enhance the process of matching patients with eligible clinical trials, enabling increased recruitment at potentially lower costs.
Recent Innovation in Clinical Trials Matching Software by Deep 6 AI
- In September 2023, Deep 6 AI launched its genomics module. The software used AI to mine disparate genetic reports to find patients with specific genetic markers in real-time and genomics data across an entire electronic medical record (EMR) system. Now, life sciences companies and healthcare organizations can precision-match patients to clinical trials based on their complete genomic and clinical profile, recruitment, feasibility, and improved trial design.
Regional Insights
North America dominated the clinical trials matching software market in 2023. The increasing growth of the acceptance of clinical trials matching software by biotechnology and pharmaceutical companies in emerging countries, the increasing number of companies pharmaceutical companies in emerging countries, the increasing number of companies investing in the deployment of clinical trial matching software, and the well-established and strong pharmaceutical industry are expected to drive the growth of the market.
In addition, the regulatory environment is beneficial to the commercialization and development of clinical trial matching software. Several software regarding AI and IT-based solutions and increasing integration of CTMS further contributed to propel the growth of the clinical trials matching software market in the region. The U.S. and Canada are the leading countries in this region.
- For instance, in June 2024, announced the launch of One Home for Sites by IQVIA in the United States. It was a new technology platform that acted as a single dashboard and single sign-on for the key tasks and systems a clinical research site needed to perform across all the clinical trials it was conducting. This advanced technology overload reduced the time site staff have to treat and recruit patients.
Asia Pacific is expected to grow at a significant rate during the forecast period. The growing improvement in healthcare infrastructure in developing countries, a rapidly growing economy, and a rising number of healthcare IT projects are expected to drive the growth of the clinical trials matching software market in the Asia Pacific region. In addition, the growing number of companies investing in research and development activities contributed to propel market growth. China, India, Japan, and South Korea are the leading countries in the region. China and India are the largest countries and most developed and established countries in the healthcare sector.
- For instance, in February 2024, the leader in evidence generation for advanced clinical trials, Signant Health, announced it has finalized the operational technical, and investments needed to support customers' compliance with China's Personal Information Protection Law (PIPL) for clinical trial data collection. The PIPL included requirements that govern how clinical research sponsors transfer and process data collection from Chinese patients.
Market Potential and Growth Opportunity
Artificial intelligence in the clinical trials matching software
AI can be used to enhance the diversity of participants, inform clinical trial eligibility criteria, and reduce sample size requirements. The AI tool Trial Pathfinder is used in electronic health record data to improve clinical trials, analyzing the overall survival risk ratio between two or more groups of patients.
Artificial intelligence is also used in multimodal imaging markers from various imaging modalities such as ultrasound, computed tomography, positron emission tomography, and magnetic resonance imaging. The clinical trials matching software uses natural language processing devices that learn both the patient RWD and clinical trial protocols. These factors are expected to accelerate the growth of the clinical trial matching software market in the coming years.
Clinical Trials Matching Software Market News
- In April 2024, a provider of clinical research solutions, WCG launched Total Feasibility, a new application on WCG's ClinSphere technology platform designed to transform the site feasibility process. The aim behind this launch was to allow the rapid development of more precise matching of sites to a study, clinical study feasibility questionnaires, reduced redundancy, more precise matching of sites to a study, and quick response times from potential sites.
- In June 2024, ICRI School of Clinical Research and Healthcare partnered with Max Healthcare and launched the Post Graduate Diploma in Advanced Clinical Research (PGDACR) program. The company said that the institution informed that this 10-month program aimed to equip students with practical training and comprehensive education in clinical research.
Market Segmentation
By Components
- Services
- Software
By End-User
- Clinical Research Organizations (CRO)
- Pharmaceutical and biotechnology Companies
- Medical Device Firms
By Deployment Mode
- On-Premise
- Web and Cloud based
Buy this Research Report@ https://www.precedenceresearch.com/checkout/2467
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308