Synthetic Control Arms Market Size and Forecast 2025 to 2034
The synthetic control arms market is gaining momentum as pharmaceutical companies adopt real-world data and advanced analytics to replace traditional placebo groups, enhancing trial efficiency, patient recruitment, and ethical standards.The rising demand for decentralized clinical trials is driving the global synthetic control arms market.
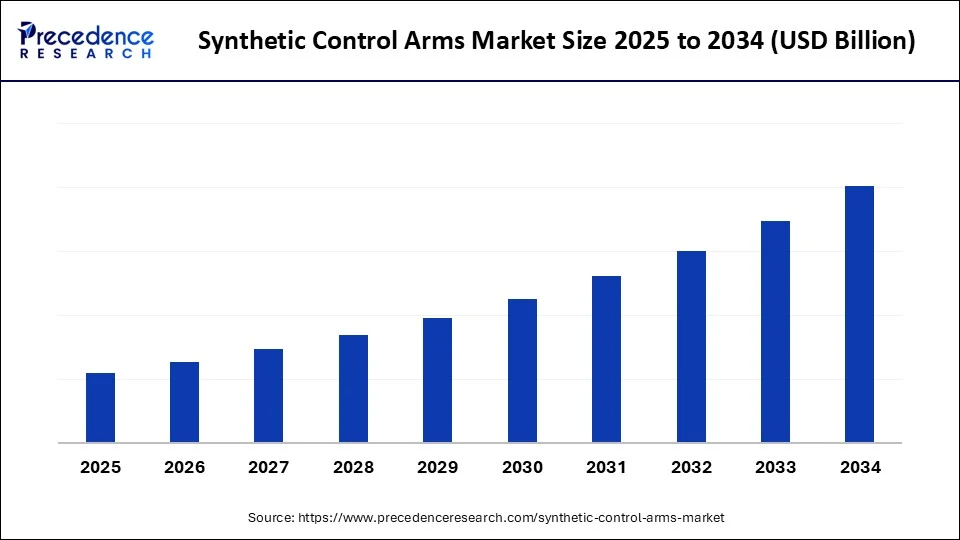
Synthetic Control Arms Market Key Takeaways
- North America dominated the global synthetic control arms market with the largest share of 58.50% in 2024.
- Asia Pacific is expected to grow at the fastest CAGR from 20245 to 2034.
- By data source, the real-world data (RWD) segment led the market while holding 53.50% share in 2024.
- By data source, the hybrid (RWD + Historical Trial Data) segment will grow at a significant CAGR between 2025 and 2034.
- By application, the oncology segment held the major market share of 47.50% in 2024.
- By application, the rare and orphan diseases segment is expected to grow at the highest CAGR between 2025 and 2034.
- By technology, the AI/ML analytics platforms segment dominated the market while holding 37.50% share in 2024.
- By technology, the simulation software segment will grow at a significant CAGR between 2025 and 2034.
- By end-user, the pharma & biotech companies segment contributed the biggest market share of 58.50% in 2024.
- By end-user, the regulatory & health technology assessment (HTA) bodies segment is expected to expand at a significant compound annual growth rate (CAGR) between 2025 and 2034.
- By trial design type, the single-arm interventional trials with SCA segment contributed the largest market share of 49.50% in 2024.
- By trial design type, the platform and basket trials using SCAs segment will expand at a significant CAGR between 2025 and 2034.
- By delivery mode, the service-based models segment led the market while holding 63.50% share in 2024.
- By delivery mode, the platform-based models segment is expected to expand at a significant CAGR between 2025 and 2034.
Market Overview
The synthetic control arms (SCA) market refers to the segment of clinical trial design and technology that involves the use of real-world data (RWD) or historical clinical data to create a virtual comparator group, rather than enrolling actual patients into a traditional placebo or control arm. These synthetic controls are statistically matched to treatment groups. They are used to improve trial efficiency, reduce patient burden, minimize ethical concerns in life-threatening conditions (e.g., oncology), and support regulatory submissions with comparative effectiveness data.
Synthetic control arms are often integrated within platform trials, adaptive designs, and decentralized trials, and their development is highly reliant on AI/ML algorithms, data aggregation platforms, and real-world evidence (RWE) analytics. Global regulatory and reimbursement landscapes are changing rapidly, with players' increased demand for evidence in regulatory value. The regulations and players are focusing on the adoption of real-world data.
- For instance, the EU Joint Clinical Assessment (JCA) has implemented standards for expansion of the HTA process and requirements.
- The U.S. FDA and EMA have started to welcome evidence from synthetic control arms, particularly in cases where RCTs are not feasible.
- Flatiron Health and Friends of Cancer Research have showcased the utilization of synthetic control arms in cutting-edge non-small cell lung cancer.
What are the Key Trends of the Synthetic Control Arms Market?
- Growing and Advancing Research in Rare Diseases: A Robust research and development institute, as it increases focus on advancing research and rare diseases and oncology, for my contribution and the rising adoption of cutting-edge technologies, including synthetic control arms.
- Technological Advancements: The advancements in technologies like artificial intelligence (AI) and machine learning (ML) are emerging as significant innovations in synthetic control arms, transforming clinical trial design, result accuracy, and efficiency.
- Efficiency Gains and Accelerated Study Timelines: The use of synthetic control arms has increased in the evaluation process of novel treatments, reducing downtime and costs compared to traditional clinical trials.
- Demand for personalized medicine: The increased demand for personalized medicine has led to the adoption of synthetic control arms for specific patient populations, tracking personalized medicine approaches.
- Regulatory Support: The ongoing regulatory acceptance and approvals for the use of decentralized clinical trials and synthetic control arms are contributing to the market growth.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Data Source, Application, Technology, End Use, Trial Design Type, Delivery Model, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
The High Cost and Complexity of Traditional Trial Operations
The high cost and complexity of traditional trial operations have increased the emphasis of researchers on the adoption of cutting-edge technologies, including synthetic control arms, for their more efficiency and cost-effectiveness. Synthetic control arm solutions leverage existing data, reducing the need for additional patient recruitment and data collection, which helps to lower the cost of trials. The first data analysis and decision-making ability of synthetic control arms make them an efficient solution for clinical trials. Additionally, synthetic control arm solution helps to simplify the trial process by reducing complexity. The growing interest in Nobel Prize developments, enhanced clinical trial processes, and the need to address ethical concerns are driving the adoption of synthetic control arms in clinical trials.
Restraint
Data Quality and Standardization Issues
Data quality and standardization issues are major factors restraining the growth of the synthetic control arms market. The reliability and effectiveness of synthetic control arms depend on the availability of high-quality, relevant historical data. The lack of standardization in data structure and format challenges the integration and analysis of data using synthetic control arms. Additionally, inconsistent data can lead to inaccurate and biased results in clinical trials.
Opportunity
Use of Real-World Data (RWD)
Leveraging real-world data holds significant potential for the synthetic control arms market, especially in the healthcare and pharmaceutical industries. Researchers are rapidly utilizing real-world data to create synthetic control arm solutions, thereby reducing the cost and complexity of traditional clinical trials. The growing need to reduce additional patient recruitment and data collection is driving advancements in real-world data solutions. Growing concerns over the efficiency it takes, and the accuracy of clinical trials are contributing to this trend. As the demand for real-world evidence grows, so does the integration of synthetic control arms into research strategies.
Data Source Insights
Which Data Source Dominate the Synthetic Controls Arms Market in 2024?
The real-world data (RWD) segment dominated the market while holding the largest share in 2024. This is mainly due to its vital role in advancing synthetic control arms in clinical trials. Real-world data helps create synthetic control arms for the development of virtual control groups, reducing the need for conventional control arms. Real-world data leverages information from various sources, including medical claims, electronic health records, and various devices, to enhance the efficiency, speed, and accuracy of synthetic control arm solutions. The growing emphasis on reducing the need for patient recruitment and data collection boosts the use of real-world data in synthetic control arms.
The hybrid (RWD + historical trial data) segment is expected to grow at the fastest rate during the projection period. The growth of the segment is attributed to the widespread use of hybrid data sources for reducing the limitations of traditional clinical trials and single-arm studies. Hybrid data sources combine real-world data and historical clinical trial data. The growing demand for more efficient and potentially more ethical clinical trial operations is driving segmental growth.
Application Insights
What Made Oncology the Dominant Segment in the Market in 2024?
The oncology segment dominated the synthetic control arms market with the largest share in 2024. This is mainly due to the increased adoption of synthetic control arms in oncology clinical trials. The air and biomarker define the patient population that is the major consumer of the synthetic control arm solutions. The increased prevalence of cancer and increased demand for personalized medicine have encouraged the adoption of synthetic control arms in oncology trials for more efficiency and accuracy. Supportive regulations, such as those from the FDA and EMA, permit the use of synthetic control arms in oncology.
The rare & orphan diseases segment is expected to grow at the fastest rate in the upcoming period, driven by increased challenges and complexity of clinical trials for rare and orphan diseases. Synthetic control arms leverage real-world data to create control groups, reducing the need for large and traditional control arms that are complex to recruit in rare disease research. The growing need for efficient and cost-effective clinical trials for these conditions is contributing to the segment's growth.
Technology Insights
Why Did the AI/ML Analytics Platform Segment Lead the Synthetic Control Arms Market?
The AI/ML analytics platforms segment led the market with a major revenue share in 2024. This is primarily due to the increased adoption of AI/ML-enabled analytics platforms for analyzing vast amounts of real-world data and historical trial data. The AI/ML analytics platforms integrate cutting-edge statistical modeling and an EML algorithm to create synthetic control groups. The increased need to improve the accuracy of these solutions and reduce the complexity of datasets are contributing to the segment growth.
The simulation software segment is expected to expand at the fastest CAGR during the projection period due to the wide adoption of this technology for the creation of virtual control groups with similar characteristics to real-world patients. This technology plays a crucial role in reducing the need for costly and time-consuming placebo or active control arms by allowing researchers to generate synthetic patient data and providing mimicked characteristics of control groups. Simulation software technology leverages advanced modeling and simulation techniques for the generation of synthetic data.
End-User Insights
Which End-User Dominate the Synthetic Control Arms Market in 2024?
The pharma & biotech companies segment dominated the market in 2024 due to the increased participation of these companies in clinical trials. The adoption of real-world data and advanced analytics in clinical trials has increased among pharmaceutical and biotech companies. These companies are increasingly adopting synthetic control arm solutions to reduce trial costs and downtime while also addressing ethical concerns in clinical trials, particularly in oncology and rare disease trials.
The regulatory & health technology assessment (HTA) bodies segment is expected to grow at the highest CAGR over the forecast period. The growth of the segment is attributed to the increasing adoption and acceptance of synthetic control arms in clinical trials. Health technology assessment (HTA) bodies, such as the National Institute for Health and Care Excellence (NICE), are the value assessors of novel health technologies, including drugs and medical devices. Health technology assessment (HTA) bodies influence the reimbursement and access decisions for novel healthcare technologies. The involvement of HTA with regulations, such as those of the European Medicines Agency (EMA), is a significant development and contributes to the acceptance of synthetic control arms in clinical trials.
Trial Design Type Insights
What Made Single-Arm Interventional Trials with SCA the Dominant Segment in the Market in 2024?
The single-arm interventional trials with SCA segment dominated the synthetic control arms market with a major share in 2024. This is due to the increased use of synthetic control arms in single-arm trials to reduce the need for traditional control groups. The challenging and unethical randomized controlled trials are major adopters of the synthetic control arms solutions to offer robust comparisons, reduce costs, and shorten trial timelines. Single-arm trials include only one control patient group to receive experimental treatments with SCA.
The platform and basket trials using SCAs segment is expected to grow at the fastest rate over the projection period, driven by the increased adoption of synthetic control arms in platform and basket trial designs. These trials are enabling efficiency and enhancing the ability to reduce challenges in traditional clinical trials. The rising need for advanced methodologies in data collection, statistical analysis, and matching techniques for external control groups is driving the use of synthetic control arms in platform/basket trials.
Delivery Mode Insights
Which Delivery Mode Segment Held the Largest Revenue of the Synthetic Control Arms Market in 2024?
The service-based models segment held the largest market share in 2024, driven by the increased adoption of synthetic control arm services for various solutions, such as creating virtual control groups in clinical trials. These services are categorized into statistical modeling, trial design support, and data provision. Service-based models leverage advanced machine learning and statistical techniques to create virtual control groups tailored to the needs of traditional control groups, driving segment growth.
The platform-based models segment is expected to grow at a rapid pace in the market over the forecast period. This is primarily due to the increased use of platform-based models for accessing high-quality and real-world data to create synthetic control arms. The platform enables the leverage and analysis of a vast database for the creation of accurate and reliable synthetic control arms. The growing need for faster, cost-effective, and more efficient clinical trials, particularly for oncology and rare disease clinical trials, is driving the use of these models.
Regional Insights
What Made North America the Dominant Region in the Synthetic Control Arms Market?
North America dominated the market by capturing the largest share in 2024. This is primarily due to the high adoption rate of this technology in the region, driven by robust healthcare infrastructure and favorable regulatory involvement. There is a high demand for cost-effective and efficient research methods that support market growth. North America has robust regulations, such as those provided by the FDA, which offer significant support for the approval of novel synthetic control arms and their use in clinical trials. The region possesses a wealth of healthcare data from various resources. This provides an abundance of resources for building and validating SCAs.
The U.S. is a major player in the regional market, driven by the high adoption of synthetic control arms in clinical trials, particularly in oncology and rare diseases. There is a high demand for efficient and ethical clinical trial operations, driving the adoption of synthetic control arms. A strong focus on technological innovations, such as advancements in data analytics and machine learning, is further contributing to market growth. Moreover, the increasing volume of clinical trials supports market expansion.
Asia Pacific Synthetic Control Arms Market Trends
Asia Pacific is expected to grow at the fastest rate during the forecast period, driven by expanding healthcare infrastructure, pharmaceutical firms, and research institutes. The increasing adoption of AI and ML, especially in clinical trials and real-world evidence studies, is contributing to market growth. Rising government investments in R&D and increasing healthcare expenditure are contributing to market growth. The increasing prevalence of various life-threatening diseases is prompting clinical trials, significantly boosting the adoption of SCAs.
- For instance, according to the IDC's worldwide AI and generative AI spending guide report, released in April 2025, Asia Pacific countries such as China and Japan are making deeper investments in AI and generative AI, with an expected reach of $175 billion by FY 2028. (Source: https://my.idc.com)
China is a major player in the regional market, contributing to growth due to the country's large patient population, expanding biotechnology industry, robust investments in R&D, and supportive government regulations. The large patient pool in China provides a wide source of real-world data for the synthetic control arms. Government investments in clinical trials are contributing to the growth of the market.
Europe Synthetic Control Arms Market Trends
Europe is a significant player in the global market. The growth of the market in the region is driven by the region's advanced healthcare infrastructure, strong regulatory frameworks, and access to high-quality, real-world data. The adoption of synthetic control arms has increased in the region to enhance the efficiency and effectiveness of clinical trials. The increasing demand for efficient and ethical clinical trials is driving market growth.
The UK is a major player in the regional market, contributing to growth due to rising advancements in data science and regulatory support. The rising number of clinical trials is boosting the adoption of synthetic control arms in the UK. Supportive regulations, such as those from the UK's MHRA, are encouraging the adoption of synthetic control arms in the country.
- In May 2025, On International Clinical Trials Day, the UK's MHRA unveiled a major shift: a consultation on using external control arms (ECAs) based on real-world data (RWD) for regulatory decisions. This move aims to leverage patient-level data collected outside clinical studies to compare the efficacy and safety of interventions in clinical trials. Stakeholders have until the end of June to weigh in.
(Source: https://healthcarelifesciences.bakermckenzie.com)
Synthetic Control Arms Market Companies

- Aetion
- Flatiron Health
- IQVIA
- Veristat
- ConcertAI
- Tempus
- Prognos Health
- Syneos Health
- Medidata (Dassault Systèmes)
- TriNetX
- Komodo Health
- Evidera (part of PPD/Thermo Fisher)
- OM1
- Clinerion
- Verana Health
- HealthVerity
- Optum Life Sciences
- BC Platforms
- COTA Healthcare
- Genentech (as early sponsor/user of synthetic arms in oncology)
Recent Developments
- In March 2025, Medidata launched the “new patient, study, and data experiences at NEXT London 2025, by leveraging AI in accelerated innovations in clinical trials.” The event brought world-leading experts and the biggest names in the life sciences industry for discussion of the most topical issues about clinical trials.
(Source: https://www.medidata.com)
- In February 2025, Indegene introduced its Cortex, a fit-for-purpose Generative AI platform that is verticalization for the life science industry. This platform leverages generative AI and large language models (LLMs) for diverse applications of life sciences, including the generation and analysis of synthetic control groups.
(Source: https://www.indegene.com)
Segment Covered in the Report
By Data Source
- Real-World Data (RWD)
- Electronic Health Records (EHRs)
- Claims and Billing Data
- Disease Registries
- Patient-Reported Outcomes (PROs)
- Historical Clinical Trial Data
- Hybrid (RWD + Historical Trial Data)
By Application
- Oncology
- Neurology
- Rare & Orphan Diseases
- Cardiology
- Infectious Diseases
- Immunology
- Others (e.g., metabolic diseases)
By Technology
- AI/ML-Powered Analytics Platforms
- Statistical Modelling & Propensity Score Matching Tools
- Data Aggregation & Integration Platforms
- Simulation Software
By End Use
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic Research Institutes
- Regulatory and Health Technology Assessment (HTA) Bodies
By Trial Design Type
- Single-arm Interventional Trials with SCA
- Hybrid Trials (Synthetic + Concurrent Controls)
- Platform and Basket Trials Using SCAs
By Delivery Model
- Service-Based Models
- Platform-Based Models
- Custom-Built Analytics Solutions
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting