What is Bioprocess Containers Market Size?
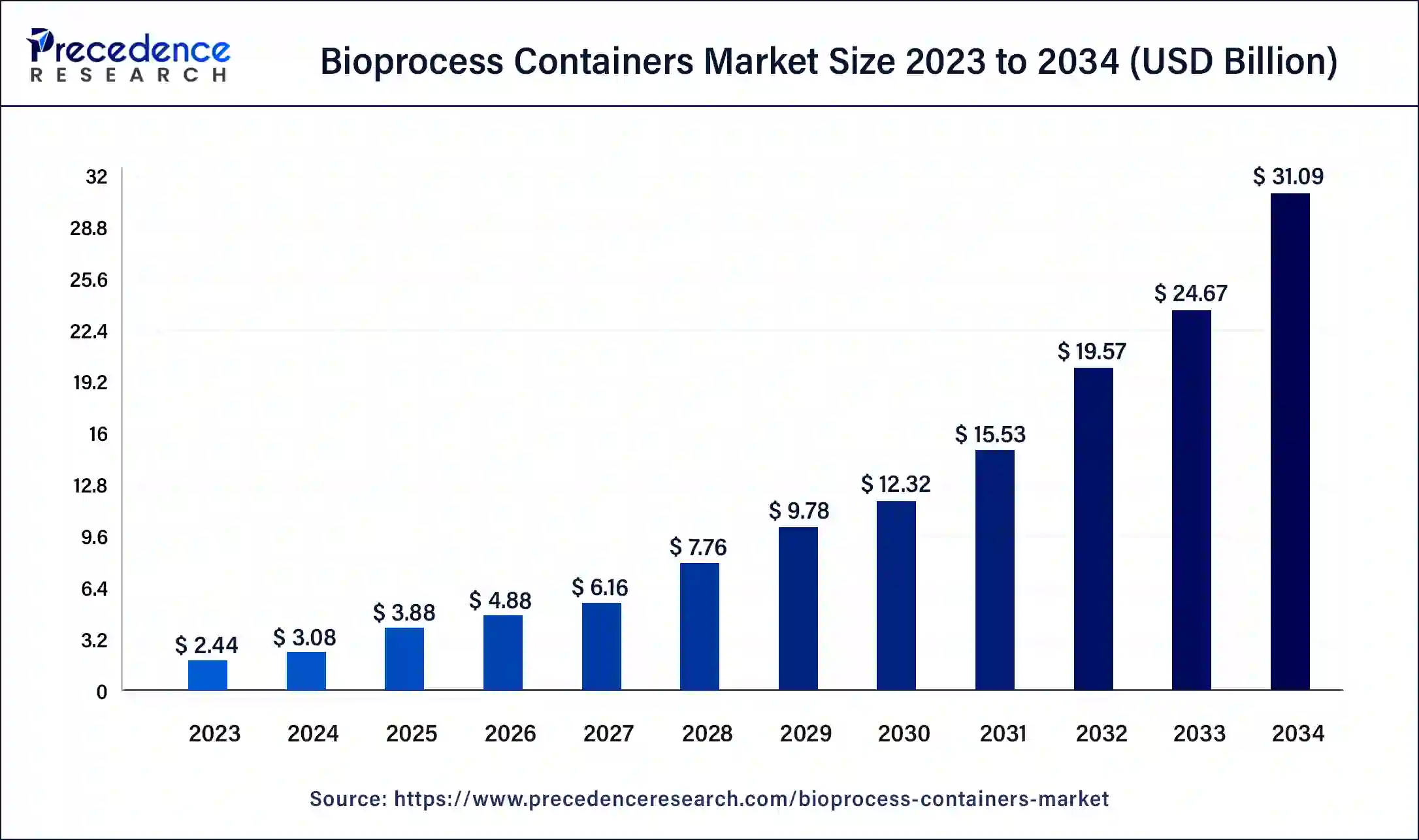
The global bioprocess containers market size is calculated at USD 3.88 billion in 2025 and is expected to reach around USD 36.63 billion by 2035. The market is expanding at a solid CAGR of 25.17% over the forecast period 2026 to 2035. The need for bioprocess containers, which are utilized in the manufacture, storage, and transportation of biopharmaceuticals, is driven by an increase in demand.
Market Highlights
- North America dominated the bioprocess containers market with the largest revenue share of 40% in 2025.
- Asia Pacific is expected to host the fastest-growing market during the forecast period.
- By type, the 2D containers segment has generated more than 46% of revenue share in 2025.
- By type, the 3D containers segment is expected to experience the fastest growth in the market during the projected period.
- By application, the upstream process segment held the largest share of the market in 2025.
- By application, the production process segment is expected to witness the fastest growth in the market over the forecast period.
- By end use, the pharmaceutical companies segment led the market in 2025.
- By end use, the biotechnology companies segment is expected to show the fastest growth in the market during the forecast period.
Market Overview
One area of the biopharmaceutical business that is expanding quickly is the bioprocess containers market. In the production of biopharmaceuticals, sterile liquids, and solutions are handled, transported, and stored in bioprocess containers. Compared to conventional stainless-steel containers, they have a number of benefits, such as being more affordable, simpler to use, and less likely to become contaminated. Comparing bioprocess containers to conventional stainless-steel systems reveals that the former is more economical. They lessen the chance of cross-contamination and cut down on cleaning and validation expenses.
The bioprocess containers market is expanding due to the growing need for biopharmaceuticals, which include cell and gene therapies, vaccines, and monoclonal antibodies. These containers are indispensable for the manufacture and storage of these goods. These receptacles provide adaptability in biopharmaceutical manufacturing procedures and are readily expandable or contracted to meet production requirements. Regulatory agencies like the FDA have offered assistance and guidelines for the use of single-use systems, such as bioprocess containers, in the production of biopharmaceuticals.
Bioprocess containers are becoming more and more dependable due to ongoing developments in design and materials, which have increased industry acceptance of these containers. The bioprocess containers market is expected to increase significantly in the upcoming years due to the growing usage of single-use technologies in the production of biopharmaceuticals. The biopharmaceutical supply chain will depend heavily on these containers as their efficiency and dependability are progressively improved by ongoing innovation and technical breakthroughs.
Artificial Intelligence: The Next Growth Catalyst in Bioprocess Containers
AI is fundamentally transforming the bioprocess containers market by enabling the shift toward "smarter" single-use systems, enhancing design customization, and optimizing supply chain logistics. AI-driven predictive modeling is increasingly used to analyze complex, high-dimensional data, allowing manufacturers to optimize the structural design and material performance of 2D and 3D bags for enhanced durability.
In production, AI-powered systems and digital twins facilitate real-time monitoring of bioreactor bags, enhancing process control and reducing, or even predicting, batch failures.
Bioprocess Containers Market Growth Factors
- The need for the bioprocess containers market is being driven by the growing need for biopharmaceuticals, which include cell treatments, monoclonal antibodies, and vaccines. These containers are utilized for a number of bioprocessing steps, including bulk drug material storage, medium preparation, and cell culture.
- The use of bioprocess containers is increasing as a result of ongoing advancements in bioprocessing technology, such as single-use systems. Compared to conventional stainless-steel systems, single-use bioprocess containers have benefits such as a lesser risk of cross-contamination, quicker turnaround times, and cheaper operating costs.
- Because bioprocess containers do not require the cleaning, validation, and maintenance that come with conventional bioprocessing equipment, they are more cost-effective. Biopharmaceutical businesses looking for scalable and adaptable production solutions may find this efficiency appealing.
- The proliferation of biotechnology and biomanufacturing endeavors worldwide, particularly in developing economies, is generating novel prospects for providers of bioprocess containers. Investments in biopharmaceutical manufacturing capacity and healthcare infrastructure are rising in these areas.
- Due to their possible environmental advantages such as lower water and energy consumption when compared to conventional bioprocessing systems single-use bioprocess containers are also seen positively. Businesses that want to comply with international environmental laws and corporate sustainability objectives may find this sustainability feature appealing.
- The safety and dependability of single-use bioprocessing systems have been acknowledged by regulatory bodies more and more, who have responded with supportive standards and guidelines. The adoption of bioprocess containers for manufacturing processes by biopharmaceutical businesses is encouraged by this support.
Bioprocess Containers MarketTrends
- The market is moving strongly toward single-use containers, which help reduce contamination risk and allow faster, more flexible production.
- Smart containers with sensors and data monitoring are becoming common, letting manufacturers track temperature, pH, and other critical parameters in real time.
- 3D rigid containers are used more for large volumes, while 2D bags remain popular for smaller or modular bioprocessing setups.
- There's a focus on sustainable materials, including bio-based or recyclable polymers, to reduce environmental impact.
- Contract manufacturers and modular facilities are driving adoption because single-use containers are easy to validate and scale quickly.
- Automation is increasing, with closed, automated container systems reducing human error and improving production efficienct.
- Regulatory trends favor closed, disposable systems, which reduce contamination risk and simplify validation for biologics and vaccines.
Market Outlook
- Market Growth Overview: The bioprocess containers market is expected to grow significantly between 2025 and 2034, driven by the shift towards single-use technology, increased development of monoclonal antibodies, and rising demand for biologics and vaccines.
- Sustainability Trends: Sustainability trends involve eco-friendly materials, recyclability and circularity, and waste reduction solutions.
- Major Investors: Major investors in the market include Thermo Fisher Scientific, Inc., Sartorius AG, Danaher Corporation, Merck KGaA, Saint-Gobain S.A., and Avantor, Inc.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 3.88 Billion |
| Market Size in 2026 | USD 4.88 Billion |
| Market Size by 2035 | USD 36.63Billion |
| Market Growth Rate from 2026 to 2035 | CAGR of 25.17% |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Type, Application, End-use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increased biologics production
Single-use bioprocess containers and other effective bioprocessing solutions are becoming more and more necessary as the biopharmaceutical industry grows. Compared to conventional stainless-steel systems, these containers have benefits such as a decreased chance of cross-contamination, quicker turnaround times, and cheaper operating expenses.
Single-use bioprocess containers save money on both capital and operating expenses by doing away with the requirement for cleaning and validation in between batches. This cost-effectiveness is especially alluring when expanding production or producing several goods at once. The increasing need for biological treatments, such as vaccinations, cell therapies, and monoclonal antibodies, has led to a need for flexible and scalable production options. The bioprocess containers market line setup and breakdown enables agile manufacturing in response to demands.
Restraint
Material limitations
The materials used to construct bioprocess containers must be compatible with the biologics being processed. This involves taking leachables, extractables, and chemical resistance into account. The materials employed should not compromise integrity or introduce contaminants while supporting sterilizing techniques like steam sterilization or gamma irradiation. Containers must be able to endure the rigors of handling, moving, and storing without losing their structural integrity.
Materials used in the production of biopharmaceuticals must adhere to regulatory requirements and guidelines, such as those set forth by the FDA in the U.S. or the EMA in Europe. Cost-effectiveness and scalability of the materials employed are significant considerations in the selection of bioprocess containers. However, they are not limitations in and of themselves.
Opportunity
Emerging biotech hubs
Biotech clusters are frequently fostered in regions with strong biotechnology and life sciences-focused universities and research institutions. The U.S.'s Cambridge/Boston and the UK's Golden Triangle (London, Oxford, Cambridge) are two examples. For biotech startups and businesses, the availability of venture finance, private equity, and government funding is crucial. Biotechnology-focused investment networks are frequently active in emerging hubs.
Being close to suppliers, pharmaceutical companies, and other biotech companies promotes knowledge sharing and cooperation, which increases a region's appeal as a biotech cluster. It is essential to have access to a skilled labor force in the bioprocessing, bioengineering, and allied industries. A strong talent pipeline is facilitated by hubs housing prestigious universities and life sciences-related vocational training programs.
Segment Insights
Type Insights
The 2D containers segment dominated the bioprocess containers market in 2025. '2D containers' in the context of the bioprocess containers market often refer to single-layer, flat film bags that are used in the production of biopharmaceuticals for the storage and transportation of liquids or powders. The purpose of these containers is to maintain product integrity and safety during the production process by offering a sterile environment for delicate biological products. They are widely used in bioprocessing for buffer solutions, media storage, and the storage of intermediate products. Because of their flat, flexible design, which facilitates effective handling and storage, they are a mainstay in the bioprocess container solutions market.
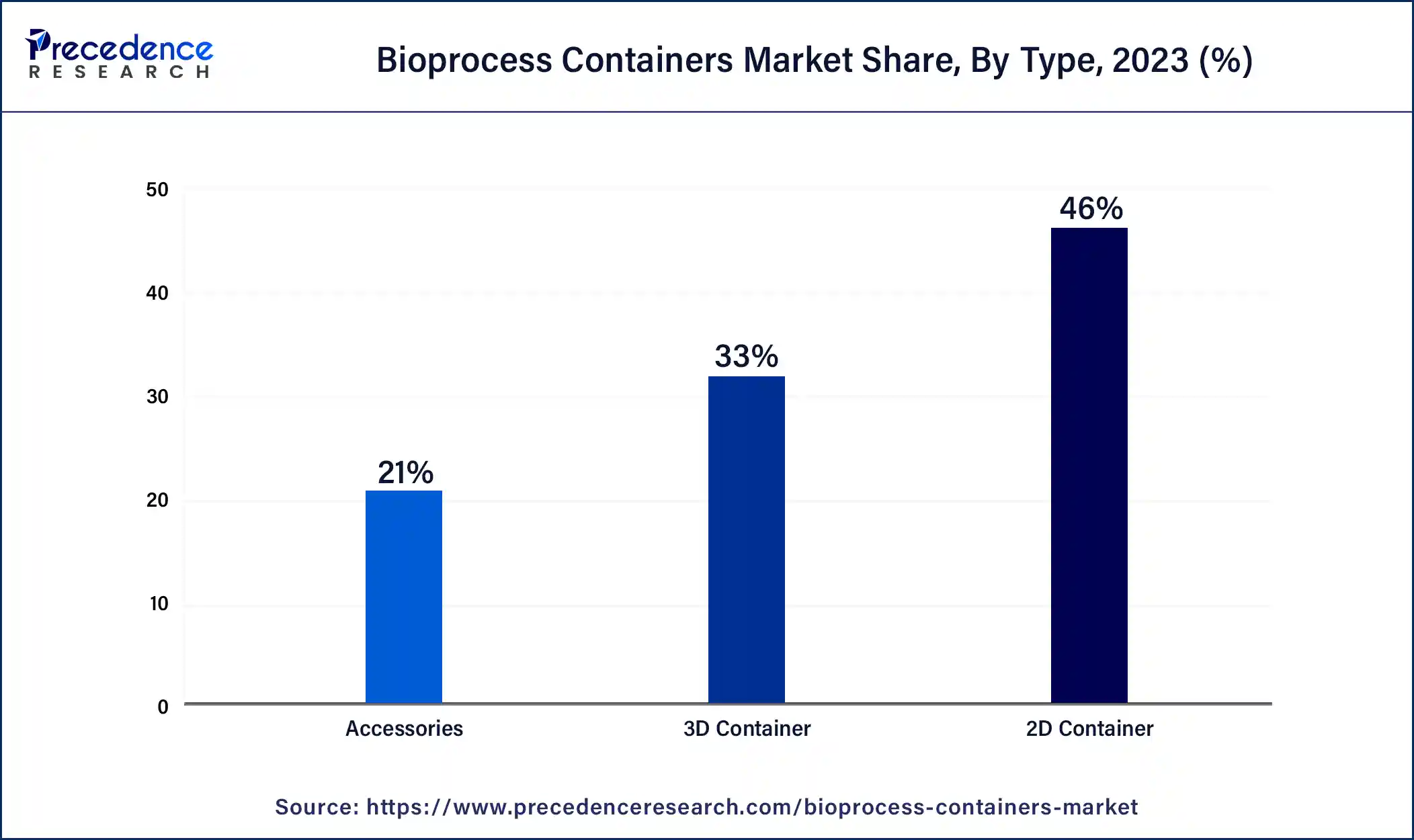
The 3D containers segment is expected to experience the fastest growth in the bioprocess containers market during the projected period. 3D containers, as used in the bioprocess container market, are made using three-dimensional printing technology. These containers have a number of benefits, including fast prototyping, adaptable designs, and the capacity to construct complicated geometries that could improve bioprocessing applications' functionality or efficiency. They contribute to flexibility and innovation in the biopharmaceutical and biotechnology sectors by being able to be adjusted to individual needs in terms of volume, form, and compatibility with different bioprocess applications. By cutting down on material waste and streamlining production procedures, this strategy also promotes sustainable practices.
Application Insights
The upstream process segment held the largest share of the bioprocess containers market in 2025. The first steps of manufacturing, which are essential for the preparation and culture of biological resources like cells,proteins, or other biomolecules, are usually included in the upstream process. Selecting premium raw materials that satisfy the particular needs of the bioprocess, including sterile plastics or films that guarantee biocompatibility and preserve the biological material's integrity.
Specialized manufacturing procedures are used in the fabrication of bioprocess containers to ensure that the containers fulfill strict specifications for sterility, integrity, and compatibility with bioprocess conditions. Strict quality control procedures guarantee that every bioprocess container satisfies legal requirements and specifications. Throughout the manufacturing process, this includes testing for sterility, integrity, and material compatibility.
The production process segment is expected to witness the fastest growth in the bioprocess containers market over the forecast period. High-quality materials are selected based on their compatibility with biologics and capacity to preserve sterility. Examples of these materials include plastics (like polyethylene and polypropylene), films (like polyethylene terephthalate), and occasionally stainless steel. For plastic components, the production process uses methods like blow molding, thermoforming, or injection molding.
Films can be laminated and extruded to make systems of flexible containers. Containers are made to comply with regulations, particular capacity needs, and various bioprocessing methods (such as chromatography and filtration). Strict quality control procedures are followed during production to guarantee that containers fulfill requirements related to sterility, robustness, and compatibility with biopharmaceutical procedures. This covers dimensional verification, visual examination, and leak testing.
End-use Insights
The pharmaceutical companies segment led the bioprocess containers market in 2025. The market for bioprocess containers is heavily influenced by pharmaceutical businesses, mainly because of their widespread use in the production of biopharmaceuticals. These containers are essential for the sterile containment, storage, and transportation of biologics,vaccines, and other pharmaceutical goods. These businesses use bioprocess containers to shorten the time it takes for pharmaceutical items to reach the market, increase flexibility, and lower the danger of contamination. Their single-use technology advancements have a major positive impact on the expansion and effectiveness of biopharmaceutical manufacturing processes. Offers disposable bioprocess containers for cell culture and downstream processing, among other bioprocessing products. Offers complete bioprocessing solutions, such as single-use equipment and bioprocess containers.
The biotechnology companies segment is expected to show the fastest growth in the bioprocess containers market during the forecast period. Because they require sterile and dependable containers for media storage, cell culture, and bioreactor operations, biotech companies are the ones driving the market for bioprocess containers. To ensure product safety and dependability, biotech companies prioritize quality assurance and adherence to regulatory norms (such as FDA and EMA) while purchasing bioprocess containers.
Single-use bioprocessing solutions, such as bioprocess containers, are being adopted by a growing number of biotech companies in order to limit cross-contamination hazards, lower cleaning and validation costs, and expedite production schedules. Their spending power and demand patterns have a big impact on the market's growth and direction for bioprocess containers, which pushes producers to develop and broaden their product lines.
Regional Insights
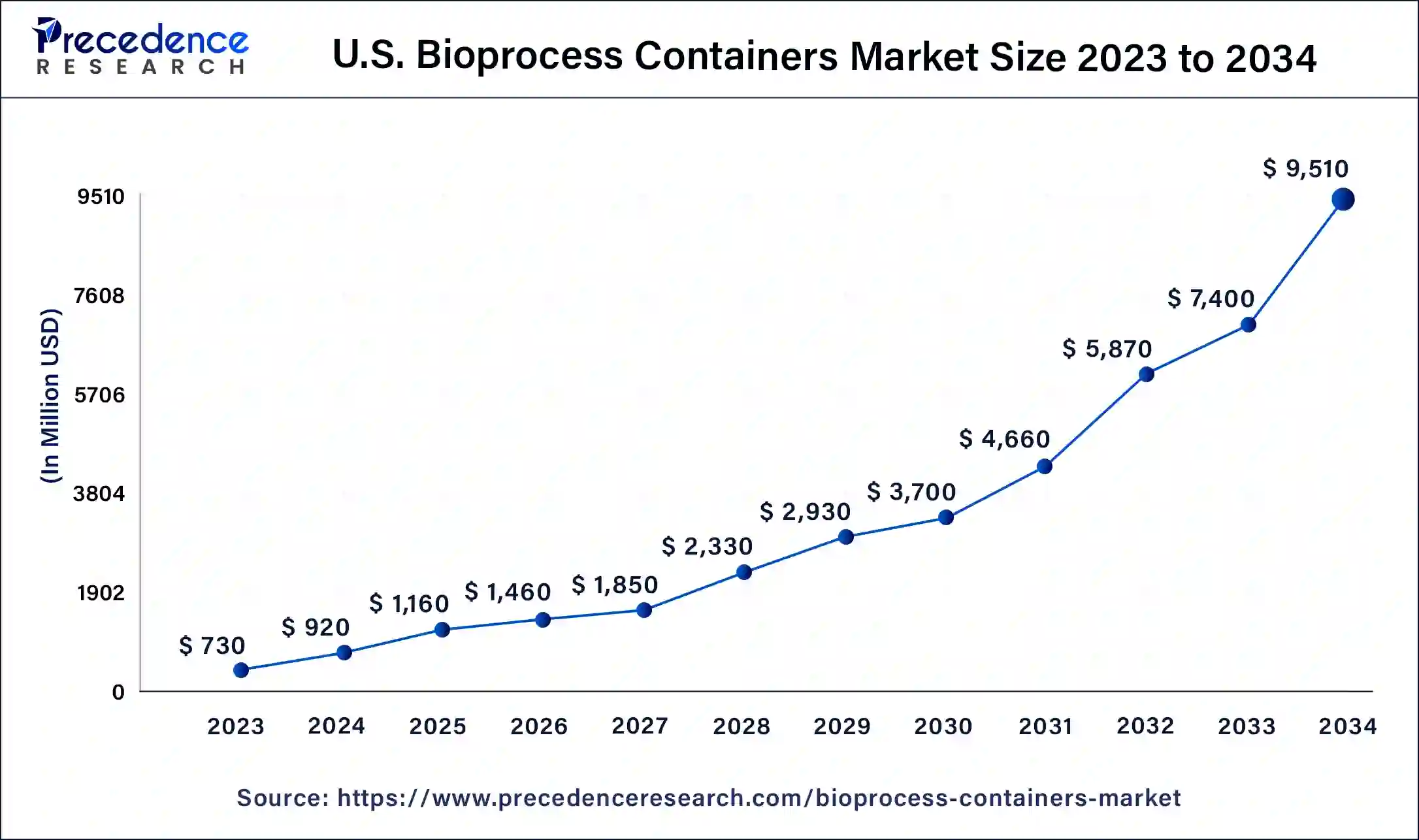
What is the U.S. Bioprocess Containers Market Size?
The U.S. bioprocess containers market size is exhibited at USD 1.16 billion in 2025 and is projected to be worth around USD 11.23 billion by 2035, poised to grow at a CAGR of 25.49% from 2026 to 2035.
North America held the largest share of the bioprocess containers market in 2025. The market for bioprocess containers in North America is expanding due to a number of causes. The need for adaptable and affordable manufacturing solutions, the development of bioprocessing technologies, and therising demand for biopharmaceuticals are some of the major forces behind this. Bioprocess containers include benefits like lower contamination concerns, more efficient processes, and easier single-use applications. Market expansion is also aided by the region's strong regulatory framework and healthcare infrastructure. All things considered, biopharmaceutical manufacturing innovation and bioprocess container adoption continue to be heavily concentrated in North America.
U.S. Bioprocess Containers Market Trends
The U.S.'s rapid expansion of CDMOs and the rise of personalized medicine are fueling the demand for modular, highly customized containers tailored for complex cell and gene therapies. Simultaneously, the market is addressing long-term viability through biodegradable materials and rigorous leachable/extractable mitigation to ensure regulatory compliance and sustainability.
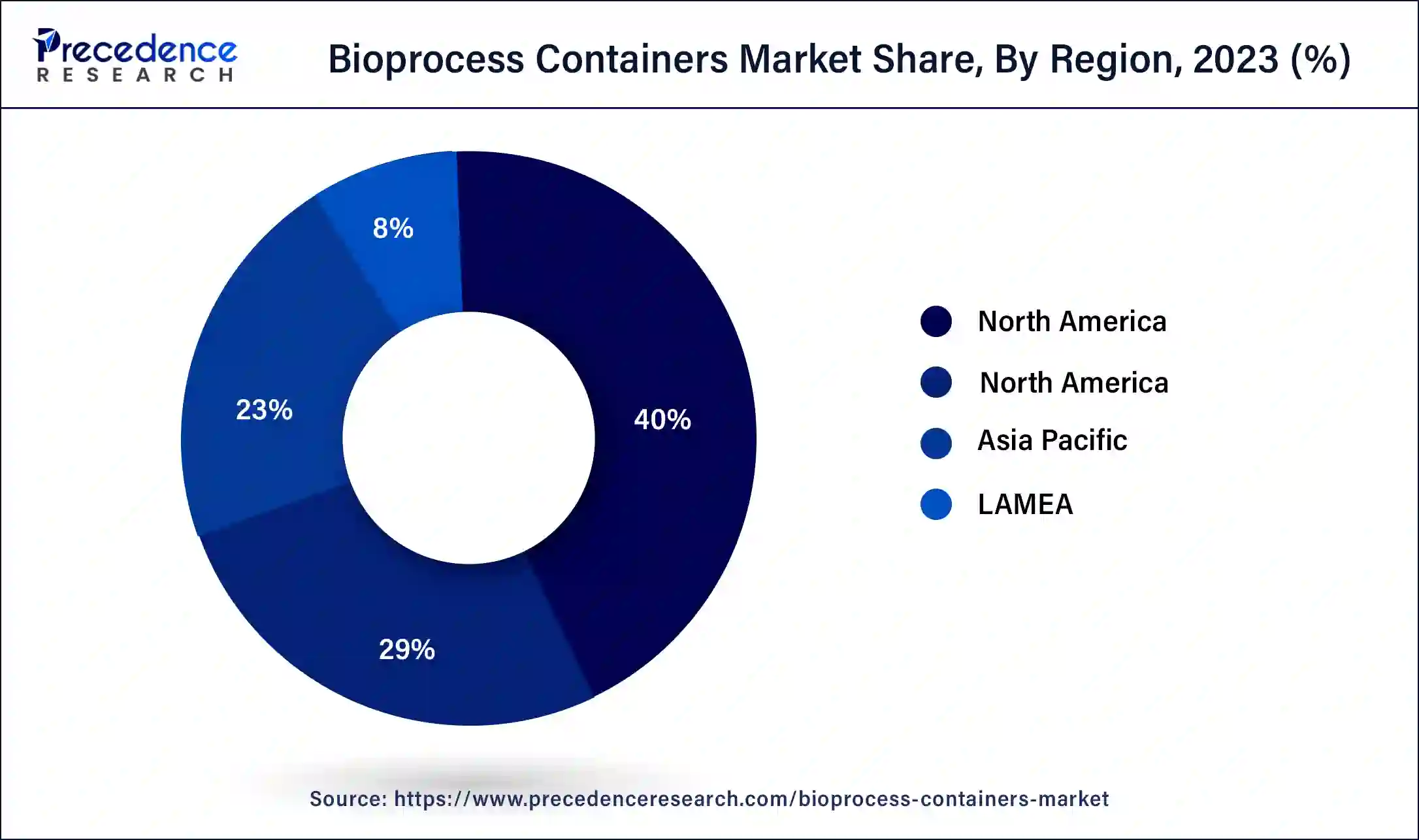
Asia Pacific is expected to host the fastest-growing bioprocess containers market during the forecast period. The Asia Pacific bioprocess containers market is expanding due to a number of factors. The region's expanding need for tailored medications, growing output of biopharmaceuticals, and improvements to the healthcare system are all important factors. The use of single-use technologies to improve efficiency and lower contamination risks in bioprocessing activities, as well as the existence of major biopharmaceutical manufacturers, are contributing factors to the market's growth. The growth of the biotechnology market in Asia Pacific is further supported by investments in research & development as well as regulatory assistance.
China Bioprocess Containers Market Trends
China's market is defined by a massive pivot toward Single-Use Technology (SUT), which now dominates upstream processing for vaccines and monoclonal antibodies. This growth is accelerated by a thriving CDMO sector that utilizes 2D and 3D containers to achieve the high flexibility and rapid scalability required for the country's expanding biologics and gene therapy portfolios.
How did Europe experience notable growth in the Bioprocess Containers market?
Europe's widespread adoption of Single-Use Technologies (SUTs) maximizes operational efficiency and sterility in the production of mAbs and personalized therapies. Backed by stringent EMA safety standards and a robust R&D infrastructure in Germany and France, the sector is increasingly prioritizing customized, modular designs for advanced regenerative medicines.
Germany Bioprocess Containers Market Trends
Germany's decisive shift toward Single-Use Systems (SUT), which are increasingly replacing traditional stainless steel to provide the flexibility required for complex, multi-product CDMO operations. This evolution is underpinned by technological breakthroughs in high-strength, multi-layer films, such as Merck KGaA's Ultimus film, which utilizes reinforced materials to minimize leakage and support the scaling of biologics and cell therapies.
Value Chain Analysis
- R&D: Research and development in bioprocess containers focuses on single-use systems, improved polymer materials, and integrated sensors to enhance efficiency, sterility, and process control. Innovations aim to support biologics, vaccines, and cell & gene therapy production.
Key Players: Leading companies in R&D include Sartorius, GE Healthcare Life Sciences, and Thermo Fisher Scientific, which are developing advanced container designs and sensor-enabled systems for scalable biomanufacturing. - Distribution to Biopharma Facilities: Bioprocess containers are distributed through specialized supply chains, logistics partners, and direct partnerships with CDMOs and biopharma companies to ensure timely delivery and product integrity. Efficient distribution is critical for high-value biologic manufacturing.
Key Players: Major distributors include Merck KGaA, Pall Corporation, and Sartorius, which provide global supply networks and logistical support to biomanufacturing facilities. - Patient Support & Services: Patient support in this context focuses on ensuring the quality and reliability of biologics produced using these containers, including training for operators and guidance on handling single-use systems. This helps maintain product consistency and safety.
Key Players: Companies providing support programs include GE Healthcare Life Sciences, Thermo Fisher Scientific, and Pall Corporation, offering training, technical assistance, and integration support for bioprocessing facilities.
Bioprocess Containers Market Companies
- Thermo Fisher Scientific Inc: Thermo Fisher Scientific is a market leader providing a wide range of single-use BioProcess Containers (BPCs), including Labtainer and Productainer systems, designed for sterile liquid handling in biopharmaceutical production.
- Saint-Gobain S.A.: Saint-Gobain Life Sciences provides an extensive range of single-use bioprocess bags, ranging from 50 mL to 3,000 L, which are constructed with proprietary multi-layer, animal-derived-component-free (ADCF) films.
- Lonza Group AG: Lonza produces Platinum UltraPAK™ Film Bioprocess Containers, which are specifically designed for the secure storage and transport of liquid cell culture media, reagents, and buffer solutions. These systems are heavily utilized within their own cGMP contract manufacturing facilities and offered to clients to provide a robust, closed-system solution for both upstream and downstream processing.
- Avantor Inc: Avantor supplies a comprehensive, "open-architecture" portfolio of 2D and 3D single-use bag assemblies and rigid, stackable containers, offering customization from 100 mL up to 3000 L.
- Danaher Corporation (Cytiva): Danaher, primarily through its Cytiva business, is a leading provider of comprehensive, scalable single-use technologies (SUT), including advanced bioreactor bags and mixing containers, designed to optimize biomanufacturing efficiency.
Recent Development
- In June 2024, Kenya is the location of NETA Auto's first flagship store. Not only is this NETA Auto's first location in Africa, but it also ushers in a new era for EVs' entry into the continent's RHD market. All of the attendees were in a vibrant and comfortable atmosphere, thanks to the opening ceremony.
- In April 2023, Merck today announced the introduction of its Ultimus Single-Use Process Container Film, which is intended to offer exceptional robustness and imperviousness for one-time assemblies utilized in liquid bioprocessing applications.
Segment Covered in the Report
By Type
- 2D Container
- 3D Container
- Accessories
By Application
- Production Process
- Upstream Process
- Downstream Process
By End-use
- Pharmaceutical Companies
- Biotechnology Companies
By Region
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting